C-19 test distribution service: submission of data on stock received
THIS CONTENT HAS NOW EXPIRED
Pharmacies participating in the NHS community pharmacy COVID-19 lateral flow device distribution service (or ‘Pharmacy Collect’ as it is described in communications to the public) must make a record of the following information when they receive cartons of test kits from their wholesaler:
- Lot Number;
- Quantity of cartons;
- Supplying wholesaler; and
- Date of receipt.
Download a form to make these records: PDF Microsoft Word
 This record must be retained for 6 months from the date on which the stock was delivered.
This record must be retained for 6 months from the date on which the stock was delivered.
The data must also be entered into the NHSBSA’s Manage Your Service (MYS) portal by the close of business on the pharmacy’s last trading day of each week.
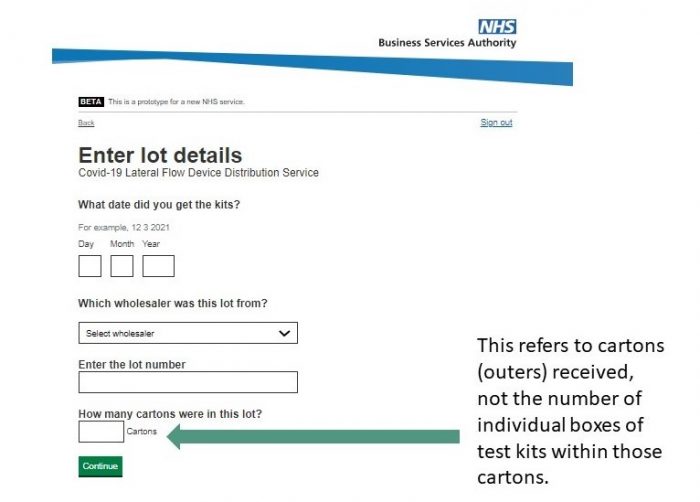
Many pharmacies have already submitted their initial data on stock received, but in some cases, there has been a misunderstanding on the quantity entered for the number of cartons received.
Some contractors have received one carton, containing 54 individual test kits, but they have entered ’54’ in response to the MYS question on the quantity of cartons in the lot, rather than ‘1’.
Contractors are asked to ensure they enter the number of cartons (outers) received in future data submissions to MYS, not the number of individual boxes of test kits within those cartons.
Read more about the service requirements and download resources









