MHRA Class 3 Medicines Recall: Prednisolone 5mg Soluble Tablets (Activase Pharmaceuticals Limited)
MHRA Class 3 Medicines Recall: Prednisolone 5mg Soluble Tablets (Activase Pharmaceuticals Limited)
Drug Alert Number: EL(25)A/54
Date issued: 15 December 2025
Activase Pharmaceuticals Limited is recalling two batches of Prednisolone 5mg Soluble Tablets as a precautionary measure due to a limited number of reports of blister pockets becoming swollen over time.
DMRC reference number: DMRC – 37820855
Medicine Details
Prednisolone 5mg Soluble Tablets PL: 28444/0276
Active ingredient: prednisolone sodium phosphate
SNOMED code: 44943411000001106
GTIN: 05060014446695
Affected Lot Batch Numbers:
| Batch No. | Expiry Date | Pack Size | First Distributed |
| 2409006 | 31/05/2027 | 30 | 06/03/2025 |
| 2409007 | 31/05/2027 | 30 | 06/03/2025 |
Background
Activase Pharmaceuticals Limited is recalling two batches of Prednisolone 5mg Soluble Tablets as a precautionary measure due to a limited number of reports of blister pockets becoming swollen over time.
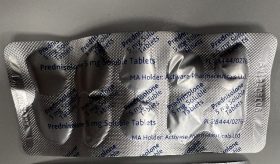
The blister pocket swelling is due to gas (carbon dioxide) build up, from gradual excipient reaction with trace amounts of moisture. The image below shows an impacted blister strip.
Advice for healthcare professionals
Stop supplying the above batches immediately. Quarantine any remaining stock and return it to your supplier, using your supplier’s approved process.
Activase Pharmaceuticals Limited have confirmed that this issue is limited to the batches listed in this notification and that no other batches are affected.
View full alert here.
Advice for Healthcare Professionals to Provide to Patients
Patients should continue to take the medication from these batches unless the blister strips exhibit signs of swelling as the quality of the tablets will not be impacted. If you have received one of the batches of medication and the blister strip exhibits swelling, return it to your dispensing pharmacy. If you have any concerns about your medication speak to your pharmacy in the first instance.
This recall is being actioned at pharmacy and wholesaler level as a precautionary measure.
Patients who experience adverse reactions or have any questions about their medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional Information
For all medical information enquiries and information on this product, please email eupvg@genreg.eu or telephone 020 72010421.
For stock control enquiries please email info@genesis-pharma.com or telephone 0207 2010400.