MHRA Class 4 Medicines Defect Information: Clexane 10,000 IU (100mg) / 1ml Syringes (Star Pharmaceuticals Ltd)
Class 4 Medicines Defect Information:
Clexane 10,000 IU (100mg) / 1ml Syringes (Star Pharmaceuticals Ltd)
Drug alert number: EL (23)A/08
Date issued: 9 March 2023
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for:
Clexane 10,000 IU (100mg) / 1ml Syringes (Star Pharmaceuticals Ltd), PLPI 20636/1822
SNOMED Code: 36564011000001106
| Batch number | Expiry date | Pack size | First distributed |
| ECD95D | 04/2024 | 10 | 07/07/2022 |
Active Pharmaceutical Ingredient: enoxaparin sodium
Clexane 10,000 IU (100mg) / 1ml Syringes (Tenolol Ltd), PLPI 30900/1997
SNOMED Code: 36564011000001106
| Batch number | Expiry date | Pack size | First distributed |
| ECI41A | 10/2024 | 10 | 07/07/2022 |
| FCA45G | 12/2024 | 10 | 13/12/2022 |
Active Pharmaceutical Ingredient: enoxaparin sodium
Brief description of the problem
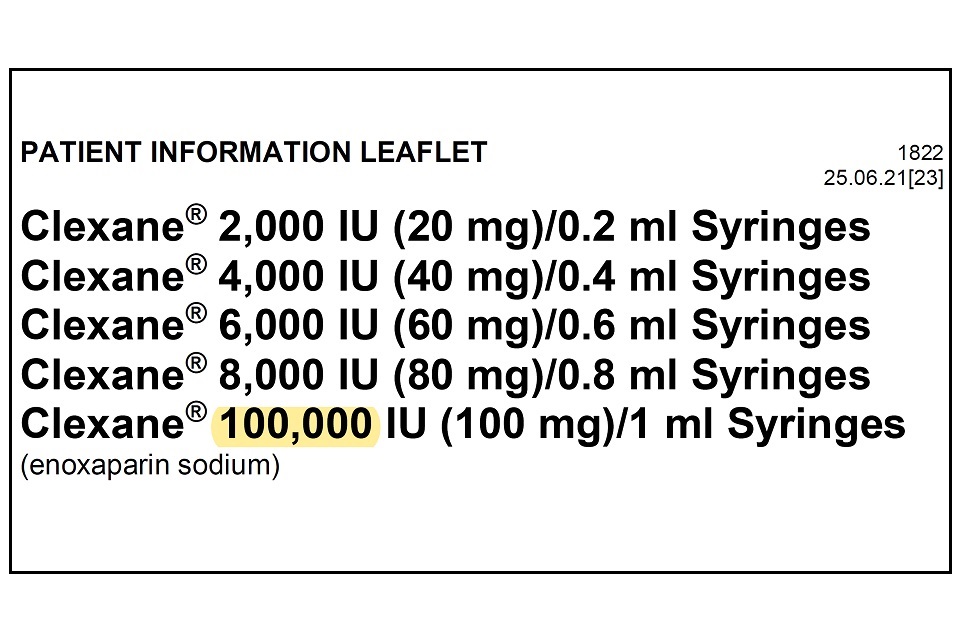
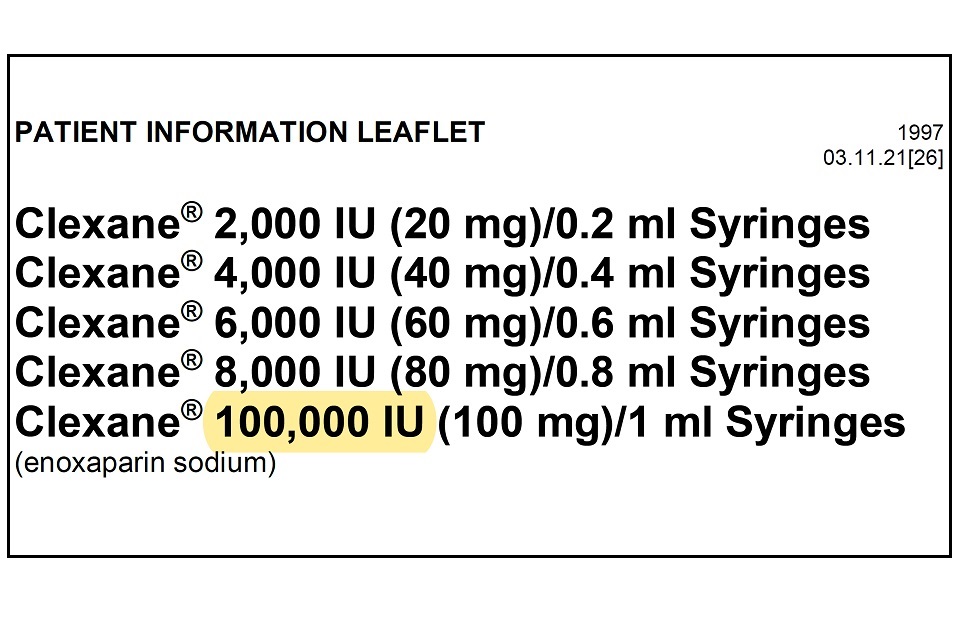
Drugsrus Limited has informed the MHRA that the Patient Information Leaflet (PIL) packaged in specific batches of Clexane 10,000IU (100mg)/1 ml Syringes contains a typographical error in the leaflet. The strength of the product listed in the leaflet header states “100,000 IU (100mg) / 1ml” instead of “10,000 IU (100mg)/1ml”. The remainder of the packaging, including the outer packaging and information on the syringe itself details the correct strength of 10,000 IU (100mg)/1ml.
A common leaflet is used for all strengths and so this error also appears in the leaflet packaged with other strengths – 2,000 IU (20mg)/0.2ml, 4,000 IU (40mg)/0.4ml, 6,000 IU (60mg)/0.6ml and 8,000 IU (80mg)/0.8ml.
Please see example image below which highlights the typographical error, which is only present on the leaflet header.
Advice for healthcare professionals
There is no risk to product quality as a result of this issue, and the affected batches are not being recalled. Healthcare professionals are advised to tell patients about the error and reassure them they have the right dose of this medicine, when dispensing subsequent batches or in discussion with patients who may raise concerns, where appropriate.
Drugsrus Limited has confirmed that all future batches will contain the correct PIL. Upon request, Drugsrus Limited will post hard copies of the updated PIL to wholesalers and pharmacies so that any remaining stock in the dispensary can be supplemented with the correct PIL information (see contact details below).
View full alert here.
Advice for patients
The Patient Information Leaflets included with these specific batches of Clexane 10,000 IU (100mg)/1ml Syringes have a typographical error. This error lists the strength as “100,000 IU (100mg)/1ml” instead of “10,000 IU (100mg)/1ml”. The correct strength is listed elsewhere on the packaging as 10,000 IU. Please continue to use the product as advised by your doctor and pharmacist. The strength and quality of the medicine itself is not impacted and this is a typographical error only.
A common Patient Information Leaflet is used for all strengths of Clexane. This means that this error also appears in the leaflet with the other strengths of Clexane – 2,000 IU (20mg)/0.2ml, 4,000 IU (40mg)/0.4ml, 6,000 IU (60mg)/0.6ml and 8,000 IU (80mg)/0.8ml.
Further Information
For more information, medical information queries or replacement PIL enquiries, please contact: recall@drugsrus.co.uk or 020 8423 3800. For stock control queries, please contact: info@drugsrus.co.uk or 020 8423 3800.








