MHRA Class 4 Medicines Defect Information: Lemsip Max Cold and Flu Capsules (Reckitt Benckiser Healthcare (UK) Limited)
Class 4 Medicines Defect Information:
Lemsip Max Cold and Flu Capsules (Reckitt Benckiser Healthcare (UK) Limited)
Drug alert number: EL (23)A/04
Date issued: 22 February 2023
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for:
Lemsip Max Cold and Flu Capsules, PL 00063/0104
| Batch number | Expiry date | Pack size | First distributed |
| AED954 | 1 DEC 25 | 16 capsules | 24 Jan 2023 |
| AED955 | 1 DEC 25 | 16 capsules | 17 Jan 2023 |
| AED956 | 1 DEC 25 | 16 capsules | 25 Jan 2023 |
| AED957 | 1 JAN 26 | 16 capsules | 17 Jan 2023 |
| AED958 | 1 JAN 26 | 16 capsules | 30 Jan 2023 |
| AED960 | 1 JAN 26 | 16 capsules | 06 Feb 2023 |
| AED961 | 1 JAN 26 | 16 capsules | 06 Feb 2023 |
| AED981 | 1 OCT 25 | 8 capsules | 04 Jan 2023 |
| AEE003 | 1 JAN 26 | 8 capsules | 06 Feb 2023 |
Active Pharmaceutical Ingredients: Paracetamol, caffeine and phenylephrine hydrochloride
Brief description of the problem
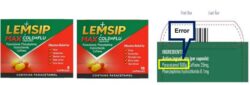
Reckitt Benckiser Healthcare (UK) Limited has informed the MHRA that a typographical error has been identified on the end flap of the outer carton of the above batches of Lemsip Max Cold & Flu Capsules. The content of paracetamol per capsule was stated as 500g (grams) instead of 500mg (milligrams). The paracetamol content of each capsule is correctly stated in Patient Information Leaflet (PIL).
Advice for healthcare professionals
Healthcare professionals should note that there is no risk to product quality and/or efficacy, therefore the affected batches are not being recalled.
Where queries are received regarding the discrepancy with the paracetamol content on the outer carton, healthcare professionals and retailers should signpost patients to Section 6 of the PIL, which states the correct content of paracetamol as 500mg (milligrams).
Section 6 of the PIL
Contents of the pack and other information
What this medicine contains:
- The active ingredients are: paracetamol 500mg, caffeine 25mg and phenylephrine hydrochloride 6.1mg
Patients should also be reminded to follow the dosing instructions on the outer carton and/or Section 3 of the PIL to ensure that the recommended dose is not exceeded.
View full alert here
Advice for patients
This notification relates to a typographical error on end flap of the outer packaging of the affected batches of this medicine. The text on the outer pack says 500g (grams) instead of 500mg (milligrams) of paracetamol.
Patients do not need to take any action as the medicine itself is not affected, and the dosing instructions on the outer carton are correct. The company has confirmed that the content of paracetamol in each capsule within the packet is correct, and that the content of paracetamol is also correctly reflected in the Patient Information Leaflet that is packaged inside the packet.
Patients should always read the leaflet that accompanies their medicines and ask a healthcare professional if they are unsure how many capsules of the medicine they should take.
Further Information
For medical information and stock control queries please contact: ConsumerHealth_GB@Reckitt.com and / or 03332005345.








