MHRA Class 4 Medicines Defect Information: Oxycodone Hydrochloride 10mg/ml oral solution (Lucis Pharma Ltd)
Class 4 Medicines Defect Information:
Oxycodone Hydrochloride 10mg/ml oral solution (Lucis Pharma Ltd)
Drug alert number: EL (22)A/49
Date issued: 29 November 2022
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for:
Oxycodone Hydrochloride 10mg/ml oral solution, PL 42176/0015
| Batch Number | Expiry Date | Pack Size | First Distributed |
| 21050004 | November 2023 | 120ml | 15/07/2021 |
| 22080005 | February 2025 | 120ml | 13/10/2022 |
Active Pharmaceutical Ingredient: Oxycodone Hydrochloride
Brief description of the problem
Lucis Pharma Ltd has informed the MHRA that there is a typographical error with text on the rear side of the outer packaging for Oxycodone Hydrochloride 10mg/ml Oral Solution.
The text incorrectly states that: ‘Each 1ml of oxycodone hydrochloride oral solution contains 1mg of oxycodone hydrochloride.’
The correct text should state: ‘Each 1ml of oxycodone hydrochloride oral solution contains 10mg of oxycodone hydrochloride.’
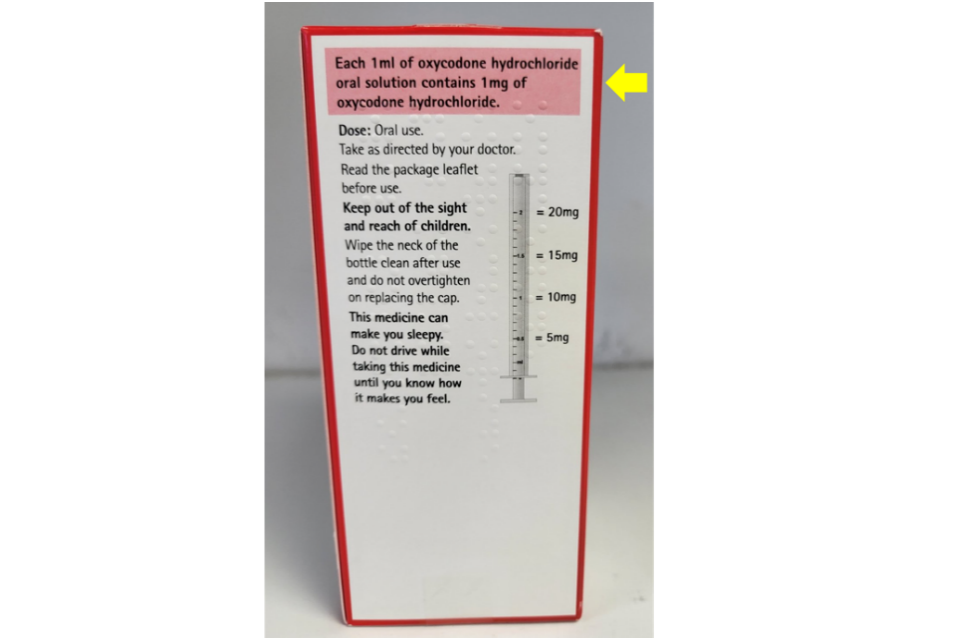
The strength of the product is printed correctly on all other sides of the outer packaging, including the label on the bottle.
Advice for healthcare professionals
Healthcare professionals should note that there is no risk to product quality and efficacy, therefore the affected batches are not being recalled.
Healthcare professionals should exercise caution when dispensing or supplying this product. Please refer to the correct information stated on the bottle label and in the Patient Information Leaflet inserted in the pack.
Advice for patients
This notification relates to a typographical error on the outer packaging of the product. The medicine itself is not affected and patients do not need to take any action.
View full alert here
For medical information and stock control queries please contact: enquiries@lucispharma.co.uk.








