MHRA Class 3 Medicines Recall: Keppra 500mg film-coated tablets (Doncaster Pharma Limited)
The MHRA has re-issued this notification as Class 3 recall based on further assessment. Please note the new actions for healthcare professionals listed within the notification. Doncaster Pharma Limited have identified an error relating to the Braille printed on the cartons on various parallel imported packs which have been repackaged by BModesto B.V.
Class 3 Medicines Recall: Keppra 500mg film-coated tablets (Doncaster Pharma Limited)
Drug alert number: EL (24)A/15
Date issued: 22 May 2024
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 3 medicines recall for: Keppra 500mg film-coated tablets (Doncaster Pharma Limited)
Company name: Doncaster Pharma Limited
Product name: Keppra 500mg film-coated tablets, PL 56830/0005
SNOMED Code: N/A
| Batch Number | Expiry date | Pack size | First distributed |
| 341173/BA | 07/2024 | 60 tablets | 21/08/2023 |
| 366686/BA | 05/2025 | 60 tablets | 21/08/2023 |
| 359701/BA | 06/2025 | 60 tablets | 21/08/2023 |
| 366621/BA | 07/2025 | 60 tablets | 21/08/2023 |
| 366635/BA | 07/2025 | 60 tablets | 21/09/2023 |
| 366635/BB | 07/2025 | 60 tablets | 21/09/2023 |
| 366101/BA | 07/2025 | 60 tablets | 06/11/2023 |
| 366635/BC | 07/2025 | 60 tablets | 06/11/2023 |
| 369420/BA | 08/2025 | 60 tablets | 01/12/2023 |
| 369421/BA | 08/2025 | 60 tablets | 01/12/2023 |
| 369421/BB | 08/2025 | 60 tablets | 25/01/2024 |
| 371089/BA | 07/2025 | 60 tablets | 01/02/2024 |
| 367482/BA | 07/2025 | 60 tablets | 01/02/2024 |
| 369420/BB | 08/2025 | 60 tablets | 01/02/2024 |
| 380087/BA | 02/2026 | 60 tablets | 05/02/2024 |
| 357677/BA | 01/2025 | 60 tablets | 12/03/2024 |
| 346651/BA | 09/2024 | 60 tablets | 02/04/2024 |
| 366635/BD | 07/2025 | 60 tablets | 08/04/2024 |
| 371089/BB | 07/2025 | 60 tablets | 11/04/2024 |
| 371016/BA | 08/2025 | 60 tablets | 11/04/2024 |
| 369420/BC | 08/2025 | 60 tablets | 11/04/2024 |
| 379799/BA | 12/2025 | 60 tablets | 11/04/2024 |
| 380087/BB | 02/2026 | 60 tablets | 11/04/2024 |
| 381719/BA | 02/2026 | 60 tablets | 11/04/2024 |
| 369421/BC | 08/2025 | 60 tablets | 23/04/2024 |
| 380089/BB | 02/2026 | 60 tablets | 23/04/2024 |
| 371016/BB | 08/2025 | 60 tablets | 24/04/2024 |
| 380089/BA | 02/2026 | 60 tablets | 24/04/2024 |
Active Pharmaceutical Ingredient: Levetiracetam
Brief description of the problem
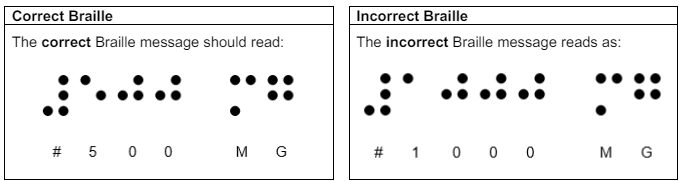
The MA holder, Doncaster Pharma Limited have identified an error relating to the Braille printed on the cartons on above parallel imported packs which have been repackaged by BModesto B.V. Approximately 70% of the packs across the listed batches have been repackaged with the Braille message on the Keppra 500mg film-coated tablets incorrectly stating strength as 1000mg.
Advice for healthcare professionals
Stop supplying the impacted batch immediately. Quarantine all remaining stock and return it to your supplier using your supplier’s approved process.
View full alert here.
Advice for patients
Specific batches of Keppra 500mg film-coated tablets have an incorrect strength printed in Braille on the outer pack, it reads 1000mg instead of 500mg. The pack contains 500mg tablets as prescribed and quality of the medicine itself is not affected by this defect. Patients are reminded to take the tablets as per the instructions from your healthcare professional and those found on the dispensing label. If there are any concerns, consult with your healthcare professional. Never stop taking medicines such as Keppra without medical advice. Suddenly stopping an epilepsy medicine may cause your seizures to start again or happen more often or last longer than before.
Patients who experience adverse reactions or have any questions about the medication, should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Further Information
For medical information and stock control enquiries please contact Doncaster Pharma on telephone: 01302 365 000.
Email quality.enquiries@doncasterpharma.co.uk or commercial@doncasterpharma.co.uk