MHRA Class 4 Medicines Defect Information: Risperidone 1mg, 2mg, 3mg tablets (Sandoz Ltd)
MHRA Class 4 Medicines Defect Information: Risperidone 1mg, 2mg, 3mg tablets (Sandoz Ltd)
Drug alert number: EL (24)A/43
Date issued: 26 September 2024
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for: Risperidone 1mg, 2mg, 3mg tablets (Sandoz Ltd)
Company name: Sandoz Ltd
Product name: Risperidone 1mg Tablets, PL 04416/0662
SNOMED Code: 38913811000001102
| Batch Number | Expiry Date | Pack Size | First Distributed |
| NK4910 | Jul-2026 | 20 | 12 Feb 2024 |
| NK4909 | Aug-2026 | 60 | 17 Jan 2024 |
Product name: Risperidone 2mg Tablets, PL 04416/0663
SNOMED Code: 20891211000001105
| Batch Number | Expiry Date | Pack Size | First Distributed |
| NL4679 | Oct-2026 | 60 | 12 Jan 2024 |
Product name: Risperidone 3mg Tablets, PL 04416/0664
SNOMED Code: 20894411000001106
| Batch Number | Expiry Date | Pack Size | First Distributed |
| MM5115 | Jul-2025 | 60 | 03 Mar 2024 |
| NE5508 | Mar-2026 | 60 | 09 Aug 2024 |
Active Pharmaceutical Ingredient: risperidone
Brief description of the problem
Sandoz Ltd. has informed the MHRA that there is missing safety information in the Patient Information Leaflet (PIL) and Summary of Product Characteristics (SmPC) for Risperidone 1mg, 2mg and 3mg and Tablets. The PIL and SmPC does not include the Adverse event of Stevens Johnson’s syndrome and toxic epidermal necrolysis in section 4.8 Adverse drug reactions for the MedDRA System Organ Class ‘Skin and subcutaneous tissue disorders’.
The missing information on the Risperidone 1mg, 2mg and 3mg Tablets PIL and SmPC is as follows:
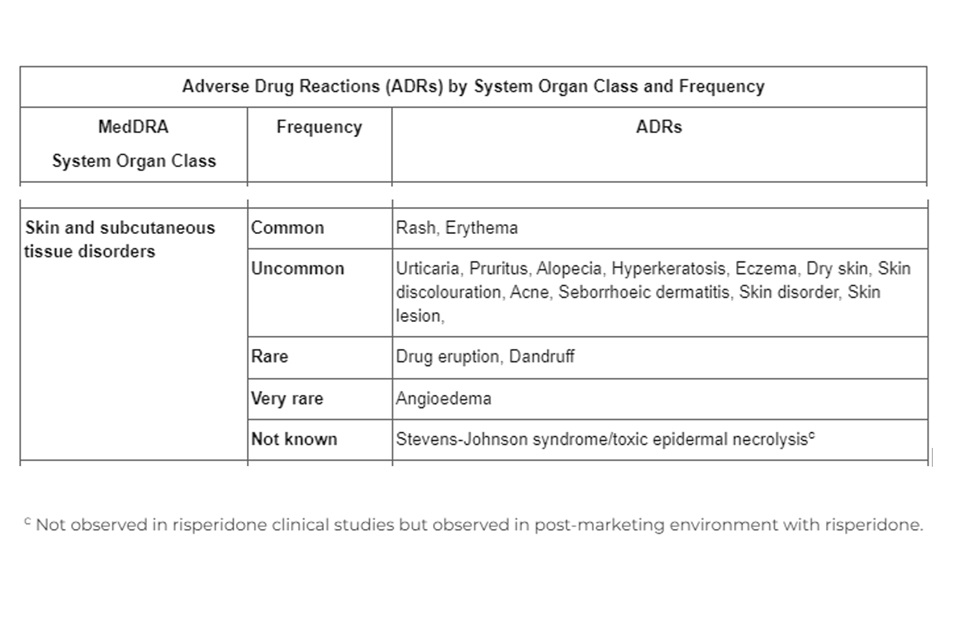
Advice for healthcare professionals
There is no risk to product quality or impact to safety of the medicines listed in this notification because of this missing information. Healthcare professionals are advised to review the content of this notification, as it provides information that is missing from the current label and take this into account when prescribing. If the medicines listed in this notification are supplied or dispensed, ensure that patients are aware of the missing information displayed above. It is important to advise patients that if patients experience any of the above symptoms they should seek immediate medical advice.
Refer to updated SmPC as follows:
SmPC, Risperidone 1mg Tablets, PL 04416/0662
SmPC, Risperidone 2mg Tablets, PL 04416/0663
SmPC, Risperidone 3mg Tablets, PL 04416/0664
Sandoz Ltd. has confirmed that all future batches will contain the correct PIL. Upon request, Sandoz Ltd. will post hard copies of the updated PIL to pharmacies so that any remaining stock in the dispensary can be supplemented with the correct PIL information (see contact details below).
View full alert here.
Advice for patients
Patients do not need to take any action. The information above is missing from the Patient Information Leaflet. The missing information, summarised in this notification, does not change or affect the quality of the product. Therefore you can safely continue your treatment. However, should you experience any adverse effects/side effects with the prescribed medication please contact your healthcare professional.
Patients who experience adverse reactions or have any questions about their medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Further Information
For medical information queries, please contact: sandozgb@EU.propharmagroup.com, Telephone: +44 1276 698 101.
For stock control queries, please contact: sales.sandoz-gb@sandoz.com, Telephone: +44 1276 698607.








