Supply of unlicensed PERT preparations
According to the Shortage of Pancreatic enzyme replacement therapy (PERT) National Patient Safety Alert (NPSA) published on 18 December 2024, Creon® will remain in limited supply until 2026 and alternatives such as Nutrizym® 22 capsules and Pancrex V® capsules and powder are intermittently available but are unable to fully cover the gap in supply.
One of the actions stated in the latest alert requires each Integrated Care Board (ICB) to have a local management plan in place to ensure patients are not left without PERT. This may include a centralised route to obtain unlicensed imports. Pharmacies are encouraged to check what local plans are in place for dealing with PERT shortages.
Due to these ongoing supply issues, community pharmacies may receive prescriptions ordering unlicensed imports of PERT. Examples of unlicenced imported PERT preparations listed in the NHS dictionary of medicines and devices (dm+d) include:
- Creon® 10000 gastro-resistant capsules (Imported)
- Creon® 25000 gastro-resistant capsules (Imported)
- Pancreaze®Delayed-Release capsules (Imported)
- Pangrol® 10,000 capsules (Imported)
- Pangrol® 25,000 capsules (Imported)
Please note: the enzyme composition of unlicensed imports may differ from UK licensed products
If an unlicensed PERT product is required, a prescription will need to be issued correctly as displayed above with ‘(Imported)’ included as part of the drug name field. This will ensure pharmacies are reimbursed for supplying the unlicensed product (provided the prescription is endorsed accordingly).
If the unlicensed PERT products listed above are not visible on prescribing systems for GPs to select (for example, because prescribing system suppliers have not ‘mapped’ across the appropriate products by using the latest dm+d extract), an FP10 paper prescription should be issued as ‘Specified PERT product (Special Order)’ or ‘Specified PERT product (Imported)’ and endorsed by the pharmacy as a non-Tariff special (see below for prescriptions endorsing requirements for non-Drug Tariff specials).
Community Pharmacy England is aware of examples where prescribers have applied different workarounds such as free-typing ‘(Special Order)’, or similar, into the dosage or additional instructions field of electronic or paper prescriptions. Such prescriptions should be referred back to the prescriber so they can be written out correctly. This is because any supplementary product information contained within the dosage area or additional instructions field is NOT read by the NHS Business Services Authority (NHSBSA) during prescription pricing. For example, if a prescription is issued for ‘Creon® 25000 gastro-resistant capsules’ with instructions included in the dosage area to supply an unlicensed special or import, the pharmacy will only be reimbursed the NHS list price (£28.25 for a pack of 100) for the licensed Creon® preparation (and not as per the endorsed invoice price for the unlicensed product supplied). Further information on supplementary product information can be found here: cpe.org.uk/dosearea.
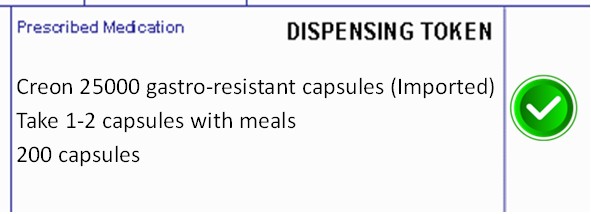
Example of EPS prescriptions issued for unlicensed Creon®
|
Incorrectly written EPS prescription for unlicensed Creon®
|
Correctly written prescription for unlicensed Creon®
|
FP10 paper prescriptions should also include the words ‘Imported’ or ‘Special Order’ as part of the prescribed product name. All paper FP10 prescriptions for unlicensed specials must be endorsed correctly and placed in the red separator for end of month submission. All paper prescriptions included in red separators are checked by an operator at the NHSBSA.
Example of FP10 paper prescriptions issued for unlicensed Creon®
|
Incorrectly written FP10 prescription for unlicensed Creon®
|
Correctly written FP10 prescription for unlicensed Creon®
|
The following endorsements are required for prescriptions for an unlicensed product(special or import) which is not listed in the Drug Tariff and has been sourced under a manufacturer’s specials/importer’s licence issued by the MHRA:
- Amount dispensed over pack size used;
- Invoice price per pack size from which the order was supplied less any discount or rebate;
- Manufacturers’/importers’ MHRA license number;
- Batch number of the product supplied;
- SP — for costs incurred in obtaining the item
Creon® SSPs
Please note, there are currently two active SSPs for Creon® capsules: SSP 060 and SSP 061. If the prescription is for more than one month’s supply, these SSPs allow pharmacies to supply the equivalent of one months’ supply. Click here for more information on Serious Shortage Protocols.
Further information
How prescriptions for unlicensed specials should be written
Dealing with product information within the dose area
Funding & Reimbursement Shorts: Handling prescriptions with supplementary product information