Dispensing an NHS prescription
Published on: 18th June 2025 | Updated on: 28th November 2025
Community pharmacy teams receive NHS prescriptions ordering a variety of different products from a range of prescribers across different healthcare settings, for example, GP practices, dental practices, hospitals, and urgent care centres. While most items prescribed on NHS prescriptions can be dispensed and reimbursed without issue, some products are not allowed to be ordered on an NHS prescription. Where an item that is not permitted on an NHS prescription is inadvertently dispensed, this would result in the item being disallowed.
This page provides practical guidance to help you determine whether an item:
- Can be supplied on the NHS
- Has been prescribed by an authorised prescriber
- Is presented on a valid NHS prescription form
- Includes the necessary prescriber endorsements
- Has been prescribed in a way that ensures correct reimbursement
Identifying whether a product is a drug or a medical device will enable you to identify whether the item can be dispensed against an NHS prescription.
Drugs (including non-medicinal products such as food supplements and cosmetics)
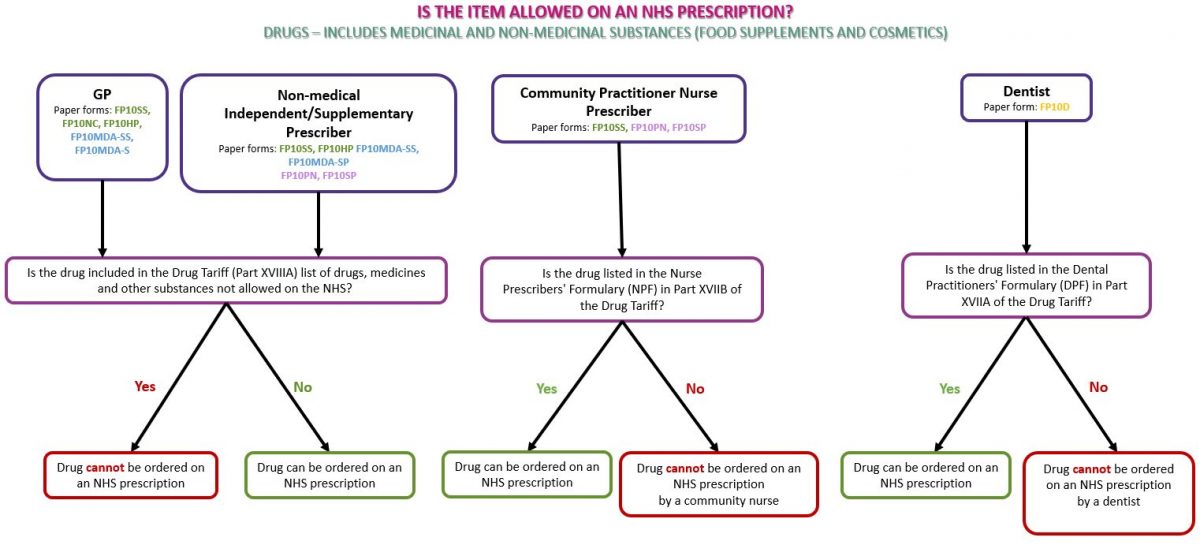
Drugs refer to medicinal products and products such as food supplements and cosmetics used for medical purposes. Subject to prescribing rights (see Prescriber type below), any drug or non-medicinal product such as food supplements or cosmetics may be prescribed on an NHS prescription if the product(s) do not appear in Part XVIIIA (drugs, medicines and other substances that may not be ordered under the NHS) of the Drug Tariff (see below)
See the flowchart below as a guide to identifying whether the prescribed drug(s) can be dispensed on an NHS prescription:
Medical device/appliance
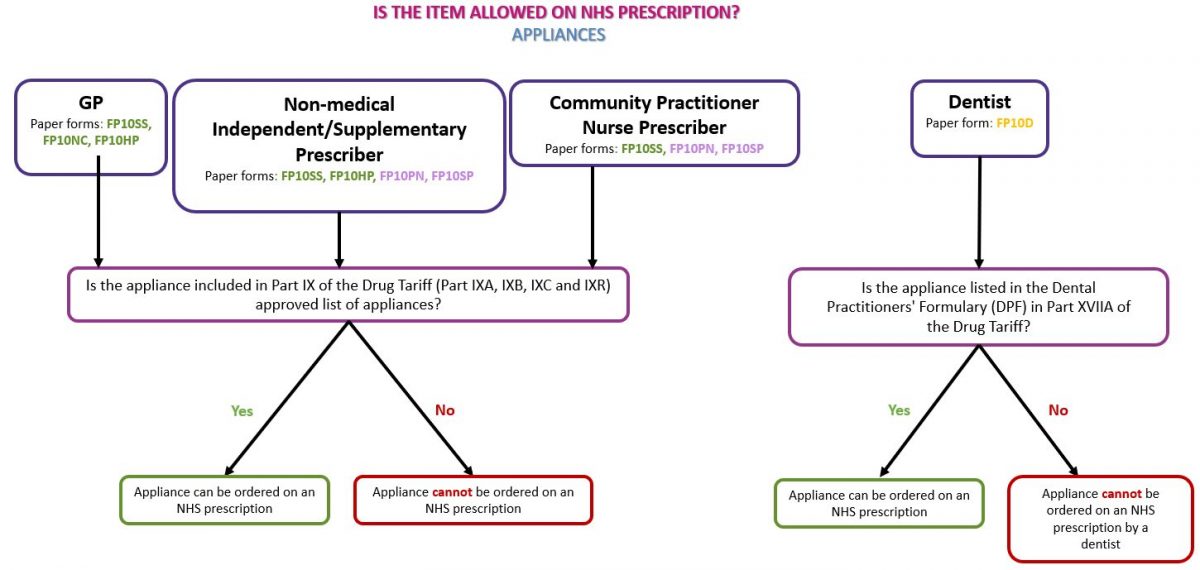
Medical devices, or appliances, can be identified by the presence of a CE or UKCA mark on the packaging of a device, the instruction leaflet and/or on the device itself (where practical). An appliance can only be prescribed on an NHS prescription if it is listed in Part IX (the Appliances section) of the Drug Tariff and must be prescribed in line with the Drug Tariff listing. See our page Dispensing Appliances for more information.
See the flowchart below as a guide to identifying whether the prescribed appliance(s) can be dispensed on an NHS prescription:
Part XVIIIA (drugs, medicines and other substances that may not be ordered under the NHS)
Part XVIIIA is the list of medicinal products which cannot be prescribed on the NHS (as set out in Schedule 1 to the NHS (General Medical Services Contracts) (Prescription of Drugs etc.)).
If a prescription for items listed in Part XVIIIA of the Drug Tariff is dispensed and submitted to NHSBSA for payment, these would be returned to pharmacy owners as disallowed and would not be passed for payment.
If you receive a prescription for an item listed in Part XVIIIA of the Drug Tariff, pharmacy teams can:
a) return the prescription to the prescriber so that an alternative product can be prescribed; OR
b) a private prescription could be issued (note, an NHS prescription cannot be used as a private prescription and a new prescription, which isn’t on an FP10, must be issued); OR
c) if the item has GSL or P status, the product could be sold to the patient over the counter.
Before dispensing an NHS prescription, pharmacy teams must check that the prescriber holds the relevant registration(s) to prescribe the item in question noting that not all healthcare professionals have the same prescribing rights, and some are limited to specific formularies.
Formulary restrictions
| Prescriber Type | Prescribing Scope |
| Dental Prescribers | Limited to items in the Dental Prescribing Formulary (Part XVIIA of the Drug Tariff) |
| Community Practitioner Nurses | Limited to items in the Nurse Prescribers’ Formulary for Community Practitioners (Part XVIIB(i) of the Drug Tariff). |
| Independent Prescribers (such as nurse, pharmacist or paramedic) | Can prescribe within their clinical competence, no formulary restrictions. |
| Supplementary Prescribers | Can only prescribe within the Clinical Management Plan agreed with the patient and Doctor. |
Note: Prescriptions issued outside these boundaries risk disallowance. For more information on the prescribing rights for the different types of prescribers including the following independent prescribers; Physiotherapists, Chiropodists/Podiatrists, therapeutic radiographer and Optometrist visit our page Who can prescribe what?
Common scenarios to check:
- A dentist ordering a non-dental formulary item.
- A nurse prescriber prescribing beyond their clinical competence.
Only certain types of prescriptions can be dispensed on the NHS. Pharmacy teams should verify the prescription form type and its validity for NHS dispensing.
- Identifying form type:
- NHS paper form types can be identified by the form type code on the bottom right of the prescription.
- EPS prescriptions include prescriber type EPS codes instead of form type codes. For more information see our factsheet Prescriber type EPS codes.
- Valid NHS prescriptions – The National Health Service (General Medical Services Contracts) Regulations state that ‘prescription forms’ and ‘repeatable prescriptions’ must be in the format required by the NHS Business Services Authority (NHSBSA). For the full specification click here.
- Controlled Drug (CD) prescriptions are subject to writing requirements such as the requirement that quantities must be written in both words and figures. For more information see Dispensing Controlled Drugs.
- Invalid forms or private prescriptions cannot be dispensed on the NHS.
For detailed guidance, see our page Prescription form validity
Certain products require specific endorsements on the prescription to be eligible for NHS supply and reimbursement. These endorsements must be applied by the prescriber and cannot be added by pharmacy teams.
Below is a list of the endorsements which only a prescriber can add to a prescription item.
| Prescriber Endorsement | What does it mean for dispensing |
| SLS | If required, this endorsement must have been made by the prescriber before dispensing. If a medicine which is found in the Selected List Scheme (Part XVIIIB of the Drug Tariff) has not been endorsed by the prescriber, the prescription would need to be returned to the prescriber for amendment. |
| ACBS | If an item which is found on the Borderline Substances list (Part XV of the Drug Tariff) has not been endorsed by the prescriber, the product can still be dispensed and will be passed for payment by NHS Prescription Services. Pharmacy staff are not required to endorse ‘ACBS’ on prescriptions. |
| FS | ‘FS’ enables prescribers to endorse item(s) to indicate to dispensers that the item(s) are to be provided to the patient free-of-charge. The FS endorsement is an item level endorsement that must be applied to each applicable item. For EPS prescribers must apply ‘FS’ using their prescribing system, it should not be manually added to the EPS dosage instructions field as this is not captured by the NHSBSA during prescription pricing. FP10 paper prescriptions containing ‘FS’ endorsed items must be placed in the relevant red separator for end-of-month submission. No prescription charge should be levied for any ‘FS’ endorsed items. For more information see Dispensing Factsheet: ‘FS’ endorsement for ‘Free supply for specified use’. |
| Either ‘OC‘, ‘CC‘ or ‘♀’ | This endorsement is the prescriber declaring that the item is to be used for contraceptive purposes. No patient charge is payable for this item. |
| Bulk Prescription | This endorsement is the prescriber declaring that the criteria for issuing bulk prescriptions have been met. No patient charge is payable for this item. |
The Selected List Scheme (SLS)
Items included in the ‘Selected List Scheme’ which can be found in Part XVIIIB (drugs, medicines and other substances that may be ordered only in certain circumstances) of the Drug Tariff, are products which can only be prescribed for the patient groups and for the purpose(s) listed in the Tariff.
Prescribers must endorse prescriptions for these products with SLS. If the SLS endorsement is missing, the prescription should be returned to the prescriber for amendment to include the SLS endorsement. Pharmacy staff cannot make the SLS endorsement themselves.
Pharmacy teams should note that regulations have been introduced to restrict the prescribing and supply of puberty suppressing hormones / gonadotrophin releasing hormone (GnRH) analogues to children and young people under 18 for the purposes of gender dysphoria / gender incongruence. From 26 July 2024 onwards, all GnRH NHS prescriptions are required to have the SLS endorsement.
The new arrangements apply to medicines that consist of or contain buserelin, gonadorelin, goserelin, leuprorelin acetate, nafarelin, or triptorelin. This includes, but is not limited to, medicines sold under the brand names: Decapeptyl®, Gonapeptyl Depot®, Salvacyl®, Prostap®, Staladex®, Zoladex®, Synarel®. Refer to our Briefing: 011/24: New restrictions on the prescriptions and supply of puberty blockers for a full list of medicines covered by the arrangements and FAQs. For more information see our page Gonadotrophin Releasing Hormone (GnRH) Prescription Regulations.
Please note that there are separate lists in Part XVIIIB of the Drug Tariff for English and Welsh prescribers to follow. Pharmacy teams should confirm they are checking the correct list before dispensing.
CC, OC or the female symbol (♀) (Free-of-charge contraceptives)
Part XVI, Clause 10 of the Drug Tariff sets out the arrangements for charging of contraceptives.
Prescriptions for the following items are automatically exempt from prescription charges:
- spermicidal gels, creams, films and aerosols
- systemic contraceptive preparations listed in Part XVI of the Drug Tariff
- contraceptive devices listed in Part IXA of the Drug Tariff.
However, there are some products, such as Co-cyprindiol 2000mcg/35mcg tablets, which are sometimes used for contraceptive purposes, but they do not appear in Part XVI. In this scenario, if the doctor has endorsed the prescription with ‘CC’, ‘OC’ or the female symbol (♀) to indicate that the item is for contraceptive use, the patient should not incur a prescription charge. Pharmacy staff cannot apply the contraceptive use endorsement themselves.
FS (Free-of-charge items)
The ‘FS’ endorsement was originally introduced in 2019 to enable prescribers to endorse a prescription to signify that items prescribed to treat a sexually transmitted infection (STI) are to be provided free-of-charge. From 1 December 2025, use of the ‘FS’ prescriber endorsement has been extended to treatments used in the management of tuberculosis (TB) and latent TB infections, including the effects of TB and the adverse effects from TB treatment.
Pharmacy teams should ensure that no prescription charge is levied for any ‘FS’ endorsed items.
There is no specific list of drugs to which the ‘FS’ prescriber endorsement can be applied. The ‘FS’ prescriber endorsement applies to any treatments used in the management of STIs and/or TB and latent TB infections, including the effects of TB and the adverse effects from TB treatment.
The ‘FS’ endorsement can only be added by a prescriber; it CANNOT be added by a patient or a pharmacist.
Community Pharmacy England has produced a factsheet that explains what the prescriber ‘FS’ endorsement is, how prescribers should apply this endorsement to electronic and paper prescriptions, and how pharmacies should correctly submit ‘FS’ endorsed prescriptions for payment. See our Dispensing Factsheet: ‘FS’ endorsement for ‘Free supply for specified use’.
ACBS (The Borderline Substances List)
Borderline substances are nutritional or dermatological products that have been specially formulated to manage medical conditions.
In certain conditions, some foods and toiletry preparations have the characteristics of drugs. A board known as the Advisory Committee on Borderline Substances (ACBS) advises on the circumstances in which these products may be regarded as drugs. Prescriptions for products recommended by the Committee should be endorsed ‘ACBS’ by the prescriber.
If an item which is found on the Borderline Substances list (Part XV of Drug Tariff) has not been endorsed by the prescriber with ‘ACBS’, this product can still be dispensed and will be passed for payment by NHS Prescription Services. Pharmacy staff are not required to endorse ‘ACBS’ on prescriptions.
Following a public consultation, Original Pack Dispensing (OPD) rules have been introduced in England to give pharmacists the flexibility to dispense up to 10% more or less of an eligible product, compared with the quantity prescribed, if it means the product can be supplied in the manufacturer’s original pack (except where this would negatively affect the patient’s clinical treatment regimen).
On 1st January 2025 changes were made to The National Health Service (Pharmaceutical and Local Pharmaceutical Services) Regulations (PLPS) to allow pharmacists to use OPD +/-10% rules for NHS dispensing.
For NHS dispensing, the PLPS amendments:
- require the supervising pharmacist to consider, using their professional judgement, whether it is reasonable and appropriate to dispense +/-10% of the prescribed amount, having regard to the benefits to patients where they are provided with products in the manufacturer’s original outer packaging. Arguably there may be a professional imperative to dispense an original pack for safety reasons (as the patient information leaflet (PIL) will be supplied) but professional judgment should be exercised to assess the suitability of applying OPD +/- 10% dispensing, as it is not expected to be appropriate in all cases.
- give the pharmacist the flexibility to supply up to 10% more or less than the prescribed quantity, if that would mean the medicine could be dispensed in the manufacturer’s original pack, and other prescription requirements are fulfilled.
All POMs, non-POMs (P medicines, GSL medicines, non-medicines) and Schedule 5 Controlled Drugs (CDs) are within scope of OPD +/-10% rules for supply against NHS prescriptions. However, there are a number of products excluded from the OPD +/-10% rules for NHS dispensing. For example, all Schedules 1-4 CDs, Part IX appliances, unlicensed specials and products classed as special containers fall outside the scope of OPD +/-10% rules. To view products in scope of the OPD rules, see our Original Pack Dispensing page.
HMR OPD exceptions
The amendments made to the HMR to allow for OPD also set out three distinct exceptions where it is recognised that full/sub packs may need to be supplied outside of the OPD +/-10% rules. The HMR OPD exceptions include:
- medicines in a form that makes it not practicable to dispense in the exact quantity ordered;
- medicines in a container that has an integral means of application or from which it is not practicable to dispense an exact quantity;
- medicines that cannot be dispensed in the quantity ordered without adversely affecting the medicine
Special container status would be granted if a product meets at least one of the three HMR OPD exceptions listed above or fulfils any of the special container criteria outlined in Clause 10B of the Drug Tariff. For special containers, pharmacy owners can supply and will be reimbursed for the nearest pack size (complete pack or sub pack) or combination of containers nearest to the quantity ordered.
It is important to note, the HMR OPD amendments also mandated full-pack dispensing of all valproate-containing medicines from October 2023. More information on this can be found here.
Further information on dispensing special containers can be found on our webpage Special containers and products requiring reconstitution.
Please see our reimbursement table below to understand how products outside of the scope of OPD and that are not special containers, are reimbursed.
Quantity reimbursement table
| Classification of product | How it will be reimbursed | How to endorse quantity dispensed |
| Medicinal products listed in Part VIIIA (excluding special containers, items requiring reconstitution or those where OPD has been utilised) | If the quantity of a prescribed item is equal to or a multiple of a listed Drug Tariff pack size, reimbursement will be based on that pack size. Otherwise the pack size will be assessed in the following order using: (i) the next largest pack size to the quantity prescribed OR (ii) the largest pack size available. Reimbursement will be pro-rata against the appropriate pack size. |
The pack size only needs to be endorsed for Part VIIIA Category C products where there are multiple pack sizes listed. |
| Medicinal products outside of Part VIIIA (excluding special containers, items requiring reconstitution or those where OPD has been utilised) | If the quantity of a prescribed item is equal to or a multiple of a pack size the NHSBSA has on their database, reimbursement will be based on that pack size.Otherwise the pack size will be assessed in the following order using: (i) the next largest pack size to the quantity prescribed OR (ii) the largest pack size available. Reimbursement will be pro-rata against the appropriate pack size. |
The pack size only needs to be endorsed where there are multiple pack sizes available. If the product is less common, the net price of that pack before discount and ex VAT should also be endorsed. |
|
Unlicensed Specials and Imports listed in Part VIIIB (except special containers)
|
The minimum quantity pack listed will be reimbursed for any amount prescribed up to the minimum quantity. Subsequent quantities will be reimbursed at the additional price per ml/g/tab/cap up to the total quantity prescribed. | No quantity endorsement required. |
| Unlicensed Specials and Imports outside of Part VIIIB (except special containers) | Reimbursement will be calculated pro rata from the declared invoice price per pack. | Amount dispensed over pack size used and invoice price per pack size used to dispense from (minus any discount or rebate), e.g.:30/30 £90/pack. Other endorsements are required for non-Part VIIIB items; please check the page opposite for full details. |
| Special containers | Where the quantity ordered by the prescriber does not coincide with that of an original pack, the contractor shall supply in the special container(s) nearest to the quantity ordered, unless there is an overriding clinical need to dispense the exact amount. Reimbursement is automatically based on the nearest number of special containers required to meet the prescription. If exactly half-way between two packs, contractors should round down. |
Contractors are required to endorse the prescription form accordingly: amount dispensed over pack size used. |
|
Items requiring reconstitution
|
When the quantity reconstituted from an original pack or packs is unavoidably greater than the quantity ordered and it has not been possible for the contractor to use the remainder for or towards supplying against another prescription, reimbursement will automatically be based on the nearest pack or number of packs necessary to cover the total quantity ordered. | Contractors are required to endorse the prescription form accordingly: amount dispensed over pack size used. |
| Appliances (all those listed in Part IX) | If the quantity of a prescribed item is equal to or a multiple of a listed pack size, reimbursement will be based on that pack size. Otherwise the pack size will be assessed from those listed in Part IX of the Drug Tariff. Reimbursement will be pro rata against the appropriate pack size. | The pack size only needs to be endorsed where there are multiple pack sizes listed in Part IX. Please note: if there is more than one pack size available for an appliance but not all pack sizes appear in Part IX, the prescription must be dispensed from and clearly endorsed as one of the Drug Tariff listed pack sizes. |
What happens if quantity is ordered by directions only?
Where a quantity is ordered by direction only, the directions should be used to calculate the quantity required to complete the course of treatment as directed. The quantity supplied must be endorsed, and NHS prescription services will reimburse based on the endorsed quantity. Please note that a month is taken to be 28 days unless indicated otherwise.
If the product is eye drops which have been prescribed by dosage instructions, e.g. two drops twice a day in left eye for two months, then the pharmacist will have to use their professional judgement to decide on how many bottles would be appropriate to meet those requirements. Reimbursement would be based on endorsement.
What to do when the required quantity has not been specified on the prescription
Missing quantity (NOT Schedule 2 and 3 Controlled Drugs):
- Confirm the required quantity with the prescriber, endorse the quantity with the letters “PC” (initial and date) in the endorsement column. Reimbursement will be based on the pharmacy team’s endorsement.
- If unsuccessful in contacting the prescriber, consider supplying enough for 5 days’ treatment and endorse the prescription with the letters “PNC” (initial and date) in the endorsement column.
Missing quantity for Schedule 2 and 3 Controlled Drugs:
- If lacking total quantity in words or figures, the pharmacist can dispense if they are satisfied that the prescription is genuine and the intention is clear. The pharmacist can amend the prescription indelibly so it has total quantity in words and figures. More information on this can be found on our Dispensing Controlled Drugs page.
- If lacking total quantity in both words and figures, the prescription must be returned to the prescriber for amendment. The prescription cannot be dispensed until amended.
For more information on this topic please email comms.team@cpe.org.uk