HRT Prescription Prepayment Certificates (PPCs)
Published on: 30th March 2023 | Updated on: 13th June 2024
In February 2023, the Department of Health and Social Care (DHSC) announced that patients in England will be able to access cheaper Hormone Replacement Therapy (HRT) for menopause through a new HRT prescription prepayment certificate (HRT PPC). From 1 April 2023, patients who are not already exempt from NHS prescription charges will be able to purchase an annual HRT PPC for the cost of two single prescription charges. The Department of Health and Social Care (DHSC) has issued guidance on the HRT PPC at https://www.nhsbsa.nhs.uk/hrt-ppc-guidance.
The HRT PPC can be used against any listed HRT medicine licensed for the treatment of menopause. The HRT PPC will be available to purchase through the NHS Business Services Authority (NHSBSA) website or from selected pharmacies.
Community Pharmacy England is supportive of the Government’s decision to make HRT medicines more accessible to patients at reduced cost, but we consider the introduction of this new prepayment certificate (PPC) specifically for HRT medicines is complex, and in some circumstances, it will not work well with existing prescription processing and pricing systems currently used in England.
The implementation of this new type of PPC will introduce additional workload and financial risks to community pharmacy teams. It may also cause difficulties for patients with HRT PPCs, unless a HRT medicine is written on a separate prescription to other prescription items for which the patient pays a prescription charge. This is because pharmacies cannot process prescriptions that include both exempt (listed HRT medicines subject to the HRT PPC) and paid (non-HRT items subject to a prescription charge) (a mixed prescription).
We wrote to Ministers highlighting our concerns about this back in November 2022 and made representations direct to the Minister for Women and the DHSC asking for all HRT items to be free-of-charge (without a prescription charge) like contraceptives. A prescription that contains an item that is free of charge and a paid item can be processed by a pharmacy. We said this would be easier to implement and more cost-effective. However, Ministers decided to press ahead with the policy.
Regulations have been introduced to seek to overcome the issue of mixed prescriptions, and Community Pharmacy England is in discussion with DHSC to ensure pharmacies are properly compensated for the time, effort and risks associated with the introduction of the HRT PPC, and in particular mixed prescriptions.
A regulatory package, the National Health Service (Amendments Relating to Pre-Payment Certificates, Hormone Replacement Therapy Treatments and Medicines Shortages) Regulations 2023, supports the introduction of the HRT PPC and was laid before Parliament on the 21 February 2023 coming into force on 1 April 2023. The package seeks to avoid such mixed prescriptions as follows:
- First, mandating single item prescribing for Drug Tariff listed HRT medicines, so that one listed HRT medicine is prescribed on one prescription, and other non-HRT items are prescribed on separate prescriptions, and
- Second, allowing contractors to refuse to dispense ‘mixed’ prescriptions – prescriptions for patients with an HRT PPC that contain both a listed HRT medicine and a non-HRT item – as well as allowing other options.
The change to the NHS (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013 allowing contractors to refuse to dispense a mixed prescription to a patient with an HRT PPC gives 3 options. Contractors may either:
- First, refuse to dispense a ‘mixed’ prescription presented by a patient – refuse to dispense both the listed HRT medicine and the other non-HRT item – and ask the patient to return to the GP for two separate prescriptions, or
- Second, either:
- dispense the listed HRT medicine (the patient or the patient’s representative signing to claim the HRT PPC exemption), or
- dispense the other non-HRT item(s) with the prescription charge(s) paid, or
- (This may be appropriate if the patient has an urgent clinical need for the listed HRT medicine or non-HRT item(s); the patient will need to obtain another prescription for any items not dispensed)
- Third, dispense both the listed HRT medicine and the non-HRT item(s) – and complete an FP57 refund form for the listed HRT medicine (there is a charge and refund for the HRT medicines, so no money changes hands – see note below) and take a prescription charge for the non-HRT item(s).
The Department of Health and Social Care is advising pharmacies to refuse to dispense ‘mixed’ prescriptions to patients with an HRT PPC, unless the patient needs one or more of the prescription items urgently. This may be appropriate for pharmacy contractors due to the financial risks with processing ‘mixed’ prescriptions for patients with an HRT PPC.
Note: If the patient is in possession of a valid HRT PPC at the time of dispensing, then only charges for the non-HRT items should be collected and the FP57 form should be completed at the same time for the HRT medicines. This will avoid the need for the pharmacy to collect and refund charges for any HRT medicines supplied at the same dispensing episode. As the prescription is marked as ‘Paid’, the NHSBSA will deduct charges for all items (HRT and non-HRT) on the prescription and the pharmacy can re-claim the charges for the HRT medicines dispensed by declaring the number of FP57 forms submitted and total value of charges to reclaim via the end of month FP34C submission form on MYS.
The PLPS Regulations have been amended so that dispensing contractors will be able to, but not be required to, refuse to supply items on a ‘mixed’ prescription where a charge exemption is being claimed based on the HRT PPC and a charge must be paid for the non-HRT item(s).
Regulations related to GPs have been amended to mandate that a listed HRT medicine must be prescribed on a separate NHS prescription to other items – that a prescriber must only order one prescription item on a prescription form or repeatable prescription that is used by the prescriber for ordering a listed HRT medicine.
‘Mixed’ prescriptions are those that contain an item from the DHSC list of HRT medicines and a non-HRT item.
NHSBSA and Patient Medical Record (PMR) systems are not designed to handle ‘mixed’ prescriptions, they are designed to handle paid (the patient pays a prescription charge) or (and this includes prescriptions subject to a PCC) exempt prescriptions. Free-of-charge items (contraceptives and STI treatment) are handled by zero-rating the medicines or prescriber endorsement and can be processed on paid prescriptions.
In some instances, a pharmacist may part-dispense or fully-dispense a ‘mixed’ prescription because a patient has an urgent need for either the HRT medicines or the non-HRT items, or both. If part-dispensed, the patient will then need to ask the prescriber to prescribe the item that was not dispensed on the ‘mixed’ prescription. If none of the items on a ‘mixed’ prescription are dispensed, the patient will need to ask the prescriber to issue separate prescriptions for HRT medicines and non-HRT items.
Risks for contractors dispensing or part dispensing ‘mixed’ prescriptions to a patient with an HRT PPC include deductions of a prescription charges from their remuneration where no charge was collected (which results in a financial loss to the pharmacy), or claiming an item as exempt where a prescription charge was collected (which results in an overpayment to the pharmacy and the additional work of dealing with NHS recovery of the overpayment, and the potential for serious allegations against the pharmacy).
The changes to the regulations to refuse a ‘mixed’ prescription apply only to patients with an HRT PPC. If a patient does not have an HRT PPC and no other exemption applies, the patient should pay the prescription charge for the listed HRT medicine and any other item in the usual way (more than one charge may apply). The patient may request, or the pharmacy could offer to complete, an FP57 refund form so that the patient may later reclaim any prescription charges, after having later purchased an HRT PPC or standard PPC. An FP57 refund form must be completed at the time of supply/charge.
Pharmacy contractors cannot refuse to dispense an HRT-only prescription. They can only refuse to dispense a ‘mixed’ prescription to a patient who has an HRT PPC and where the prescription includes both a listed HRT medicine and another non-HRT item.
The Government has decided that the cost of an HRT PPC will be equivalent to two single prescription charges in England. From 1 May 2024, a single prescription charge is £9.90 per item and an HRT PPC will cost £19.80. An HRT PPC is valid for 12 months from the start date and covers an unlimited number of listed HRT medicines.
The list of HRT medicines covered by the HRT PPC will be published in Part XVI of the NHS Drug Tariff from April 2023. The up-to-date HRT medicines list will also be available on the HRT PPC application page and the NHSBSA help with health costs page.
The quickest way for patients to buy an HRT PPC is online at www.nhsbsa.nhs.uk/hrt-ppc. It is optional for pharmacies to sell PPCs. Community Pharmacy England recommends that patients visit the NHSBSA website to identify and purchase the PPC that is most suited for their needs.
A standard 3-month or 12-month PPC applies to all medicines prescribed on NHS prescriptions whereas an HRT PPC only applies to certain HRT medicines only.
With an HRT PPC, patients could save money if they pay more than two HRT prescription charges within 12 months. Patients who purchase an HRT PPC will continue to pay for any chargeable non-HRT items (i.e. prescribed items that do not appear on the Drug Tariff HRT list). If a patient is also prescribed other chargeable items in addition to their HRT medicines, a standard 3-month PPC will benefit those who expect to have 4 or more items in 3 months whereas a 12 month-PPC will benefit those with 12 or more items a year.
Patients who choose not to purchase any PPC and who are not exempt from paying prescription charges for any other reason, will continue to pay applicable charges for all chargeable items. For example:
- Bijuve 1mg/100mg capsules – 1 prescription charge – Bijuve is a combined HRT preparation that contains estradiol and progesterone in the same capsule. Bijuve would attract only one prescription charge.
- Elleste Duet 1mg tablets – 2 prescription charges – Elleste Duet 1mg is a continuous sequential HRT preparation that contains a combination of two different tablets; one contains estradiol only and the other contains both estradiol and norethisterone. Elleste Duet would attract two prescription charges as it comes in a combination pack containing two different items.
It is the patient’s responsibility to check that they have the right exemption or certificate in place when claiming free NHS prescriptions and signing the declaration on the prescription form.
Before purchasing an HRT PPC, patients are advised to check if:
- they are eligible for free NHS prescriptions using the eligibility checker: nhsbsa.nhs.uk/check. For more information on see Community Pharmacy England’s page Exemptions from the prescription charge
- their medicine is covered by the HRT PPC. For a list of eligible HRT medication, visit: nhsbsa.nhs.uk/hrt-ppc-medicines
- a 3 or 12- month standard PPC is more suitable. A standard PPC covers all NHS prescriptions, not just HRT medicines. Visit: nhsbsa.nhs.uk/ppc
Pharmacy teams can help to raise awareness with patients about the savings they can make with PPCs by displaying posters in their pharmacy. A HC20 poster for standard PPCs and HRT PPC posters, can be ordered from the Primary Care Support England (PCSE) online portal. Pharmacies will need to login to their PCSE Online accounts and place the order for the posters. For any enquiries, the PCSE Customer Support Centre can be contacted via telephone on 0333 014 2884.
Any time a patient makes a declaration that they are exempt from paying an NHS prescription charge, pharmacy staff must ask them to sign a declaration and produce evidence. Pharmacies must advise the person claiming exemption from prescription charges, where evidence is required but not provided, that the NHS undertakes checks to verify that such persons are eligible for free prescriptions. This is a legislative requirement of terms of service The National Health Service (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013 under paragraph 7. Where a patient is unable to provide proof of exemption the patient should mark the appropriate exemption box and pharmacy staff should mark the “Evidence not Seen” box on the back of the prescription with a cross. Alternatively, the patient can pay the applicable charges for the listed HRT items and request an FP57 form to claim a refund later.
Once purchased online, the HRT PPC can be printed, saved as PDF or patients can take a screenshot of the certificate and present this at the pharmacy. Patients can also request a hard copy of their HRT PPC which NHSBSA will send by post.
From Thursday 29 June 2023, the NHSBSA has enabled functionality to allow pharmacies to sell all types of Prescription Prepayment Certificates (PPCs) through the Manage Your Service (MYS) portal. The selling of PPCs through the MYS portal is an optional, unfunded activity for pharmacies. Patients can purchase PPCs directly from the NHSBSA website, and this is the recommended route to help them identify the PPC that is right for them.
Where possible, Community Pharmacy England recommends that patients are signposted to NHSBSA website to purchase a PPC that is most suited for their needs.
- Patients can purchase an HRT PPC online at nhsbsa.nhs.uk/hrt-ppc
- A standard (3 or 12-month) PPC can be purchased from nhsbsa.nhs.uk/help-nhs-prescription-costs/nhs-prescription-prepayment-certificate-ppc
From June 2023, the Department of Health and Social Care (DHSC) has updated its definition of Hormone Replacement Therapy. The June 2023 Drug Tariff defines HRT as follows:
‘Hormone Replacement Therapy (HRT) is the replacement of female sex hormones oestrogen and progesterone in women to control symptoms of the menopause. Some medicines that are converted or break down into oestrogen, progestogen or androgen hormones are prescribed for relief of menopausal symptoms. For the purposes of the HRT PPC these are included within this definition of HRT. Types of HRT include:
- combined oestrogen plus progestogen HRT – for women who still have their uterus
- oestrogen only HRT – for women who have had a hysterectomy
- tibolone – acts as a combined hormone therapy.
To be included on the list below (and therefore within scope of the HRT PPC) the products must be:
(1) HRT as defined above; and
(2) licensed for the treatment of menopausal symptoms in the UK.
Please note: there can be variation in the licensing of different medicines containing the same active substance’
The list of HRT medicines covered by the HRT PPC will be published in Part XVI of the NHS Drug Tariff. The up-to-date HRT medicines list is available on the HRT PPC application page and the NHSBSA help with health costs page. Community Pharmacy England (formerly PSNC) has updated its HRT medicines list* to reflect the changes taking effect from June 2023. *Please note: you may need to hard refresh (press Ctrl+R) to refer to the most up-to-date version.
The HRT medicines list has been compiled following consultation with the Medicines and Healthcare products Regulatory Agency (MHRA), NHSBSA and the NHS England Menopause Clinical Reference Group. Examples of products that are NOT covered by the HRT PPC include Black Cohosh, Citalopram, Clonidine, Duloxetine, Escitalopram, Evening Primrose Oil, Gabapentin, Norethisterone, Pregabalin, Sertraline, Testosterone and Venlafaxine. Please note this is not an exhaustive list of items not covered by the HRT PPC about which patients may ask.
The list will be updated as new HRT medicines licensed for the treatment of menopause become available in the UK or as existing HRT medicines are discontinued from the UK market. If a product that meets the criteria is missing from the HRT medicines list please contact dhsc.publicenquiries@dhsc.gov.uk.
HRT may be prescribed for indications other than the symptoms of menopause. The HRT PPC is valid for any listed HRT medicines, regardless of the patient’s clinical indication or gender. The annual HRT PPC covers an unlimited number of listed HRT medicines licensed to treat the symptoms menopause.
If patient is not exempt from prescription charges and does not have a valid HRT PPC at the point of dispensing, the patient would pay the applicable number of charges for HRT medicines and the prescription would be marked as ‘Paid’. For example, for Femoston 2/10mg tablets the patient would pay two prescription charges. The patient should also be given a FP57 form at the time of dispensing. Once the patient has obtained their proof of exemption for e.g. an HRT PPC, this can be presented at a pharmacy alongside the FP57 form within 3 months and a refund for the charges paid can then be issued to the patient.
Contractors opting to sell the HRT PPC do not need to declare the number of HRT PPCs sold on the FP34C. The NHSBSA obtain the figures for the number of HRT PPCs sold and total value of the sales from your MYS portal activity.
You do not need to send any payment received for HRT PPC’s sold to the NHSBSA. The NHSBSA will deduct the monthly total value from your monthly payment based on the HRT PPCs sold via MYS.
The suite of FP10 prescription forms and the FP57 receipt and refund claim form are being updated to reflect the change.
A. FP10 and tokens
To support the introduction of the HRT PPC, FP10 forms and EPS tokens have been updated to include a new exemption category ‘W’ for “HRT only prescription prepayment certificate”.
In addition, the patient information page has been updated providing information on reporting suspected side effects to medicines and medical device incidents to the MHRA Yellow Card scheme: https://yellowcard.mhra.gov.uk/ The image below shows reverse of the existing and revised FP10 forms.
| Current version FP10SS1219 | New version FP10SS0522 |
 |
 |
The new versions of FP10 forms will have the 0522 identifier for e.g. FP10SS0522 or FP10DT0522. The existing form version is FP10SS1219. The only prescriptions which are not changing are FP10PCDNC and FP10PCDSS. Revised versions of FP10 prescription forms and tokens versions will be put into circulation from the beginning of April 2023. Once older versions of dispensing tokens have been used up, pharmacy teams should obtain updated dispensing tokens directly from Primary Care Support England (PCSE) via the pharmacy stationery online ordering portal.
Prescribers and dispensers are encouraged to continue to use the old forms until stocks run out. As the old form versions do not have the HRT PPC exemption reason, a temporary workaround has been put in place for patients who hold a valid HRT PPC to tick box ‘F’ for the standard “Prescription prepayment certificate” instead. This workaround MUST only be used for prescriptions containing ONLY items from the HRT medicines list. Procedures will be in place at NHSBSA to prevent patients using this workaround from receiving penalty charges unnecessarily.
B. FP57 receipt and refund form
The new versions of FP57 forms will have the 0423 identifier i.e. FP570423. The existing form version is FP571008. The DHSC has confirmed that the new FP57 form will be available soon. The printing of new FP57 forms will commence after the new versions of FP10’s and EPS tokens are printed. Updated FP57s can be obtained directly from Primary Care Support England (PCSE) via the pharmacy PCSE Online supplies ordering portal.
There will be a period of transition where pharmacies can continue issue the older versions of the FP57s forms until stocks run out.
The FP57 has been updated to allow pharmacists to record the number of charges paid for items on the HRT medicines list separately from other prescription items, and to address some outstanding required amendments e.g. Universal Credit and naming of the war pension exemption
The following notable changes have been made to the FP57 receipt and refund claim forms:
- Different parts of the FP57 form will be numbered instead of alphabetical. For example, Part A, B, C, D, E and F of the current FP57 form will be replaced with Part 1, 2, 3, 4, 5 and 6 respectively.
- The new FP57 now includes an additional row in Part 1 in which to record the charges collected for listed HRT medicines separately from any other charges collected for items not on the HRT medicines list.
- Part 4 (previously Part D) had been updated to include details of the issuer (the Ministry of Defence) of the war pension exemption.
- Part 5 (previously Part E) has been updated to align more closely with the letters used on the FP10 prescription form/token.
- Part 5 (previously Part E) now includes a new exemption box ‘W’, to enable patients to select when claiming a refund for listed HRT medicines.
- Part 5 (previously Part E) now includes exemption box ‘U’, to enable patients to claim a refund when included in an award of Universal Credit and meeting criteria.
| Current FP571008 | New FP570423 |
 |
 |
Pharmacists can issue the patient (or representative) with an FP57 form at the time the patient pays the applicable charges if:
- a patient (or representative) requests one for any reason
- the patient (or representative) is unsure whether they a patient is unsure whether they are entitled to free prescriptions; or
- the patient (or representative) has applied or will be applying for a prescription charge exemption certificate or prepayment certificate (3-month PPC, 12-month PPC or HRT PPC); or
- the patient (or representative) is waiting for a paper certificate to arrive at the time they need their items
Please note if a receipt is required by a carer or representative, for example to show that the charge was paid, a till receipt may be sufficient proof. Refund claims must be made within 3 months from the date the charge was paid and the FP57 was issued. If more than 3 months have elapsed between the time the charge was paid and the FP57 form is presented for payment, the charges cannot be refunded unless the patient has a form LIS04(P) from the NHSBSA.
An FP57 form should only be issued at the time the prescription charge is paid by the patient. The FP57 form cannot be issued at a later date. Part XVI Clause 7.5 of the Drug Tariff states ‘Only issue a FP57 Prescription Receipt and Refund claim form at the time the prescription charge is paid. If the patient or their representative is persistent in their request and they have grounds why they do not have a prescription refund and receipt, they should be advised to write to NHSBSA, Help with Health Costs, Bridge House, 152 Pilgrim Street, Newcastle Upon Tyne, NE1 6SN or ring 0300 330 1343, to explain why they were unable to obtain an FP57. Their Review Section will examine the claim and will either send a payment directly to the patient or advise the patient why they do not qualify.’
Old FP57 claim procedure
If a patient plans to claim a refund for an HRT item on the grounds that they held a valid HRT PPC that applied at the point of dispensing, or they were considering applying for a backdated HRT PPC and the new FP57 is not available the following steps should be followed:
- The patient would pay usual charges for all HRT and any non-HRT items and the prescription would be marked as ‘Paid’;
- An ‘old’ FP57 form must be completed on the date the charge was paid;
- The contractor or an authorised member of staff should complete Part A in full:
- Enter patients name and address as it appears on the prescription form;
- Stamp the ‘Dispenser’s stamp’ box;
- Complete all parts of section i to iii;
- Contractor or an authorised member of staff must annotate with ‘Listed HRT’ and initial the annotation to confirm the changes paid were for listed HRT items;
- A sperate ‘old’ FP57 form should be issued for any non-HRT items.
- There must be no alterations to the amounts or the quantities in Part A (sections i, ii and iii). If a mistake is made, then start again with a new form and cross through the discarded form in such a way that it cannot be used and write, “CANCELLED” diagonally across it
- Once the patient obtains their HRT PPC, this can be presented at a pharmacy alongside the FP57 form within 3 months and a refund for the charges paid can then be issue to the patient
- The pharmacy providing the refund would complete the FP57 form and declare the total number of completed FP57 forms and total amount of charges refunded on the FP34C form via MYS.
Note: If the patient is in possession of a valid HRT PPC at the time of dispensing, then only charges for the non-HRT items should be collected and the FP57 form should be completed at the same time for the HRT medicines. This will avoid the need for the pharmacy to collect and refund charges for any HRT medicines supplied at the same dispensing episode. As the prescription is marked as ‘Paid’, the NHSBSA will deduct charges for all items (HRT and non-HRT) on the prescription and the pharmacy can re-claim the charges for the HRT medicines dispensed by declaring the number of FP57 forms submitted and total value of charges to reclaim via the end of month FP34C submission form on MYS
New FP57 claim procedure
If a patient plans to claim a refund for an HRT item on the grounds that they held a valid HRT PPC that applied at the point of dispensing, or they were considering applying for a backdated HRT PPC, the following steps should be followed when using the new FP57 form:
- The patient would pay usual charges for all HRT and any non-HRT items and the prescription would be marked as ‘Paid’
- The new FP57 must be completed on the date the charge was paid
- The contractor or an authorised member of staff should complete Part 1 in full:
- Enter patients name and address as it appears s on the prescription form;
- Stamp the ‘Dispenser’s stamp’ box;
- Complete all parts of section i to iii for the listed HRT medicines and/or non-HRT items;
- Strike through any boxes not applicable i.e. if the patient is only paying for a listed HRT medicine strike through the line for ‘Item(s) not on Drug Tariff HRT only PPC list’.
- There must be no alterations to the amounts or the quantities in Part 1 (sections i, ii and iii). If a mistake is made, then start again with a new form and cross through the discarded form in such a way that it cannot be used and write, “CANCELLED” diagonally across it
- Once the patient obtains their HRT PPC, this can be presented at a pharmacy alongside the FP57 form within 3 months and a refund for the charges paid can then be issue to the patient
- The pharmacy providing the refund would complete the FP57 form and declare the total number of completed FP57 forms and the total amount of charges refunded on the FP34C form via MYS
Note: If the patient is in possession of a valid HRT PPC at the time of dispensing, then only charges for the non-HRT items should be collected and the FP57 form should be completed at the same time for the HRT medicines. This will avoid the need for the pharmacy to collect and refund charges for any HRT medicines supplied at the same dispensing episode. As the prescription is marked as ‘Paid’, the NHSBSA will deduct charges for all items (HRT and non-HRT) on the prescription and the pharmacy can re-claim the charges for the HRT medicines dispensed by declaring the number of FP57 forms submitted and total value of charges to reclaim via the end of month FP34C submission form on MYS
Submission of FP57
On the MYS FP34C declaration, you must tick to confirm if you are sending any F57 forms. Contractors will then be asked to declare the number of FP57 forms submitted and enter the total amount refunded. The completed FP57 forms must be placed at the top of your prescription bundle for submission to the NHSBSA alongside any EPS tokens for non-payment and paper Repeat Authorising (RA) forms.
Changes are being made to prescribing and dispensing systems to accommodate the introduction of the new HRT PPC.
The Government has confirmed that a digital solution will be introduced as soon as possible to prescribing systems to automate the issuing of listed HRT medicines as single-item prescriptions. However, IT solutions won’t be in place for prescribers to issue separate prescriptions from 1 April 2023. Until auto-separation of listed HRT medicines is achieved, prescribers will be required to manually issue prescriptions for all listed HRT medicines as single-item prescriptions (i.e. separate from all other prescription items, including other listed HRT medicines).
As of March 2023, DHSC has not provided any clear indication on the timescales required to achieve auto-separation. When the HRT PPC proposals were first raised, Community Pharmacy England warned the DHSC that changes to prescribing systems have been slow in the past and that all suppliers need plenty of advance notice to fit new IT development plans into their roadmaps. As suppliers have been given very short notice of the changes required to prescribing systems, Community Pharmacy England is concerned that ‘mixed’ prescriptions may continue for some time.
Community Pharmacy England will continue pressing DHSC on this to ensure that prescribing systems can be updated as soon as possible. Any progress on the work to achieve auto-separation of prescriptions for HRT listed medicines will be updated on the ‘Prescribing systems’ section of cpe.org.uk/supplierlist.
Coding has been released to dispensing system suppliers to updating PMR systems with the new HRT PPC exemption category. In EPS, the new message code or Electronic Reimbursement Message (EREM) code ‘0020’ ’for “HRT only Prescription pre-payment certificate”, will be available for dispensers to select before an EPS claim is submitted for payment for prescriptions with HRT items only dispensed to patients with a valid HRT PPC.
Over time, some PMR systems may choose to introduce logic to support dispensing processes for example by identifying listed HRT medicines on prescriptions to prompt the patient if they hold a valid HRT PPC. In the future some systems may even assist with calculating for the best PPC options for patients depending on what items they are being prescribed across their prescriptions. PMR suppliers may consider adding such development plans onto their roadmaps based on the direct feedback suppliers receive from their customers (pharmacy teams) see: cpe.org.uk/reportIT.
DHSC has recommended development work on PMR systems be completed ahead of HRT PPC implementation date of 1 April 2023. Most suppliers are working to accommodate these timescales, and it is expected that majority of, if not all, suppliers will have the new HRT PPC exemption category available for selection on PMR systems from 1 April 2023. Where dispensing systems have not been updated a temporary workaround will be in place whereby letter ‘F’, EREM code ‘0007’ for “Prescription prepayment certificate” should be selected.
Suppliers received summary guidance from DHSC in February 2023 relating to the HRT PPC Drug Tariff change. Suppliers had requested that DHSC, and the NHS shares detailed guidance with them six months ahead of time in the future relating to future significant Drug Tariff changes that impact PMRs, so that there is adequate time for planning, development, testing and PMR guidance changes and so that other planned system development work has less interruption.
The level of supplier work required can vary depending on how the system is set-up. Some suppliers will be able to schedule for all their users to receive the new HRT PPC option on the same day (e.g. 1 April 2023). Other suppliers may include the new category code within a new release alongside other updates or as part of a phased rollout. Phased rollouts across the estate might mean that some pharmacy teams have visibility of the new category code before the 1 April 2023 go live date whereas others may receive the update after.
For some systems, this is an entry into a configuration file that can be done one day and be available on all their customers’ PMR systems the next. For others, though, it is a new release and rollout can take time. System suppliers would therefore need to work backwards from the go-live date to ensure they are ready in time and their estate has the new exemption. Phased rollouts across the estate could mean that some contractors have the visibility and ability to use this exemption before the go live date
Where dispensing systems have not been updated with the HRT PPC exemption category, a temporary workaround will be in place whereby the pre-existing exemption reason ‘F’ for “Prescription prepayment certificate” EREM code ‘0007’ should be selected.
Due to the complexities of checking for exemption against specific medicines, RTEC will not be available for confirming whether a patient has a valid HRT PPC. Although DHSC states that changes to RTEC will be made as soon as possible, Community Pharmacy England believes the RTEC system and technology would likely need to be rebuilt from the ground up for it to be able to assist with HRT PPC exemption handling. This is because the RTEC system is focussed on identifying an exemption at the patient and prescription form level rather than identifying an item-level exemption. Community Pharmacy England continues its participation in the RTEC steering group which helps to set out future RTEC plans – along with NHSBSA and DHSC representatives. Updates regarding any RTEC developments can be viewed on Community Pharmacy England’s RTEC webpage.
Prescriptions for listed HRT medicine(s) only
The steps below will guide you through the process to follow when presented with an EPS prescription for a listed HRT medicine only:
- Check if the patient has a valid HRT PPC and that their prescribed HRT medicine(s) is covered by the HRT PPC
- If the patient holds a valid HRT PPC, advise the patient to sign and date the reverse of the EPS token and select exemption reason ‘W’ for “HRT only prescription prepayment certificate” (if using an older version of EPS token advise the patient to select code ‘F’ for “Prescription prepayment certificate”)
- Dispensers should apply the “HRT only prescription prepayment certificate” option (EPS category code ‘0020’ equivalent to ‘W’ on paper prescriptions and tokens) on the electronic message. If the new code ‘W’ has not yet been configured into your PMR system, dispensers should select the pre-existing standard “Prescription prepayment certificate” option (EPS category code ‘0007’ equivalent to ‘F’ on paper prescriptions and tokens)
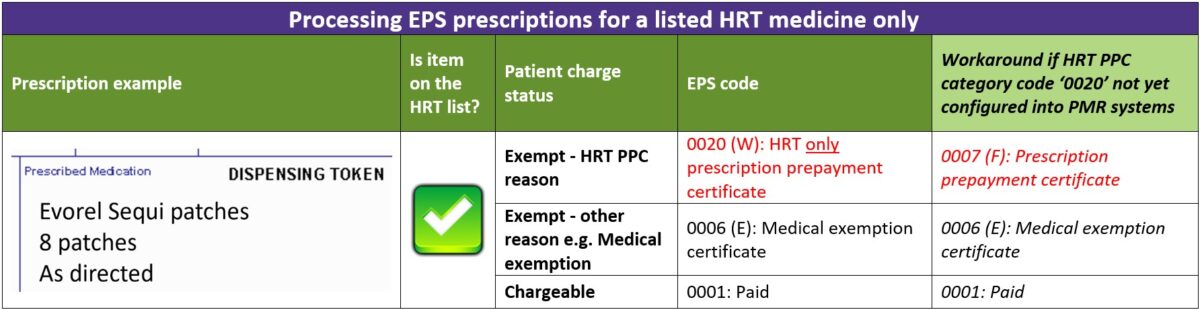
The table below explains the different EPS category codes (including a workaround option) that can be selected when dispensing an electronic prescription containing ONLY items from the HRT medicines list
‘Mixed’ prescriptions with both listed HRT medicine(s) and non-HRT items
See below steps for processing of ‘mixed’ EPS prescriptions where there is an urgent need for either the listed HRT medicines or non-HRT items, or both.
1. ‘Mixed’ prescriptions – only listed HRT medicine(s) dispensed
The steps below will guide you through the process to follow if a patient with a valid HRT PPC has an urgent need for the listed HRT medicines(s) ordered on a ‘mixed’ prescription
- Check if patient has a valid HRT PPC and that their prescribed HRT medicine(s) is covered by the HRT PPC
- If the patient holds a valid HRT PPC, advise the patient to sign and date the reverse of the EPS token and select exemption reason “HRT only prescription prepayment certificate” (if using an older version of EPS token advise the patient to select code for “Prescription prepayment certificate”)
- Dispensers should apply the EPS option “HRT only prescription prepayment certificate” on the electronic message (EPS category code ‘0020’)
- If the “HRT only prescription prepayment certificate” option has not yet been configured into your PMR system, dispensers should select the pre-existing standard “Prescription prepayment certificate” option (EPS category code ‘0007’ equivalent to ‘F’ on paper prescriptions and tokens)
- The non-HRT items should be marked as ‘Not Dispensed’ by endorsing ‘ND’
- The patient should request the prescriber to re-issue a separate prescription for the non-HRT items not dispensed. Alternatively, the pharmacist can contact the prescriber on the patient’s behalf to obtain a new prescription for the non-HRT item(s) not dispensed.
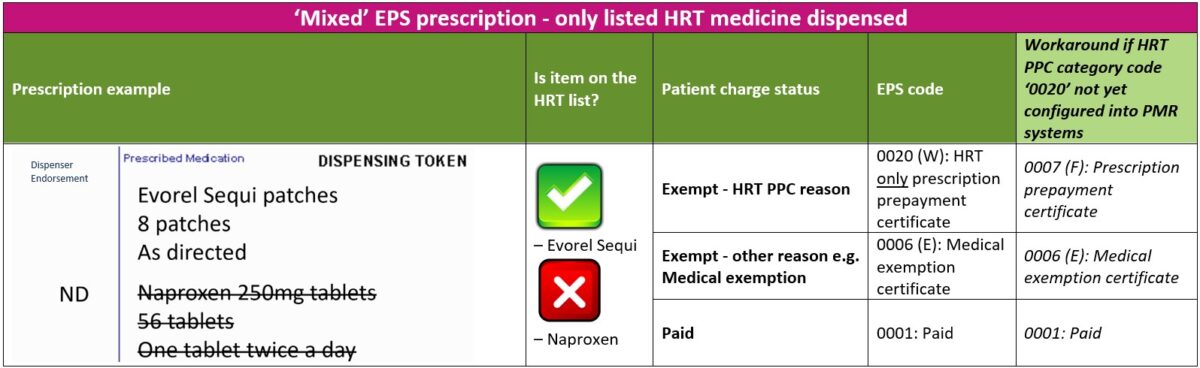
The table below explains the different EPS category codes (including a workaround option) that can be selected when dispensing an electronic prescription for a patient with a ‘mixed’ prescription.
2. ‘Mixed’ prescriptions – only non-HRT medicine(s) dispensed
The steps below will guide you through the process to follow if a patient with a valid HRT PPC has an urgent need for the non-HRT items ordered on a ‘mixed’ prescription
- Provided the patient is not exempt from paying prescription charges for any other any reason, the patient should be asked to pay the required number of charges for the urgent non-HRT items dispensed
- Patient should be asked to mark the reverse of the EPS token with the correct number of charges paid for the non-HRT items dispensed. The patient should NOT select exemption reason ‘W’ for “HRT only prescription prepayment certificate”
- Dispensers should apply the ‘Paid’ EPS category code ‘0001’ on the electronic message
- All listed HRT medicines should be marked as ‘Not Dispensed’ by endorsing ‘ND’
- The patient should request the prescriber to re-issue a separate prescription for the listed HRT medicines not dispensed. Alternatively, the pharmacist can contact the prescriber on the patient’s behalf to obtain a new prescription for the listed HRT item(s) not dispensed
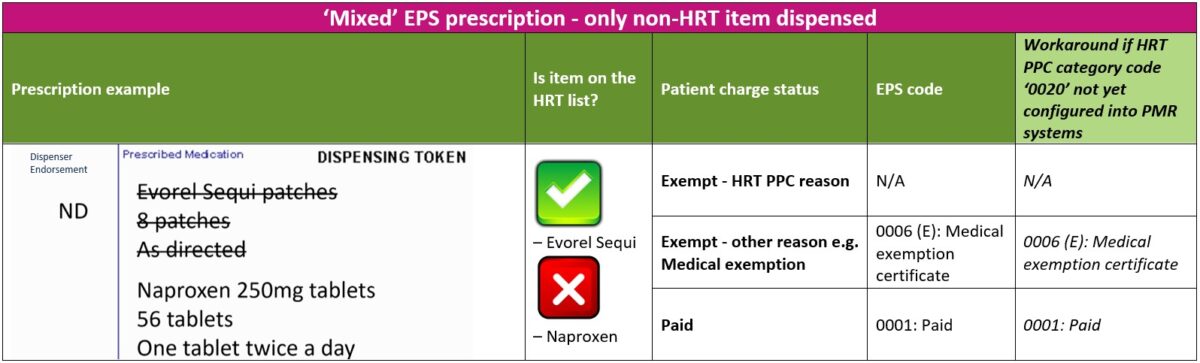
The table below explains the different EPS category codes (including a workaround option) that can be selected when dispensing an electronic prescription for a patient with a ‘mixed’ prescription where the HRT item is not dispensed.
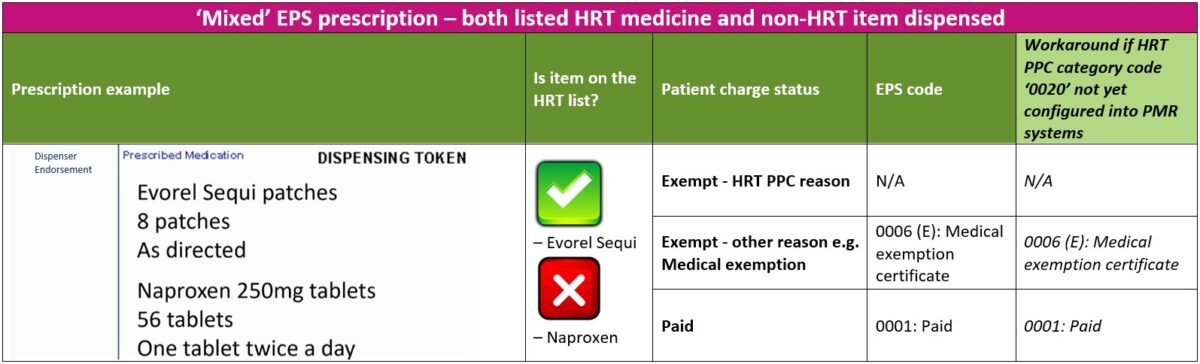
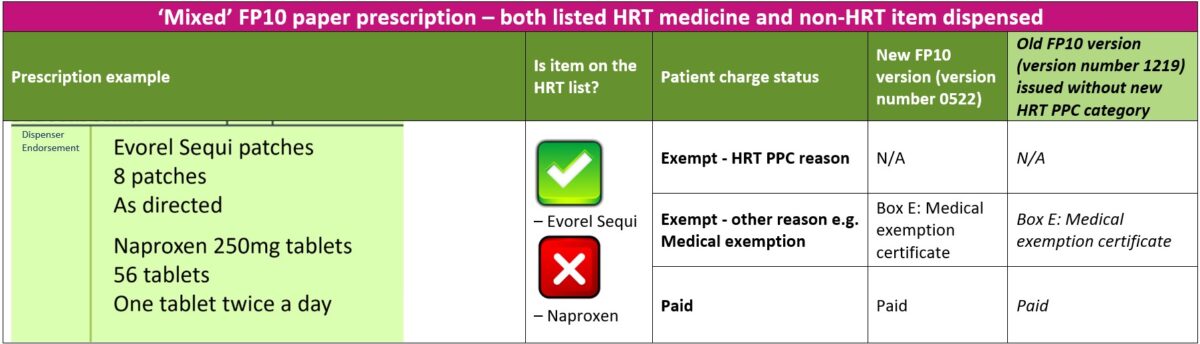
3. ‘Mixed’ prescriptions – both listed HRT medicine(s) and non-HRT items dispensed
The steps below will guide you through the process if a patient has urgent need for both HRT and non-HRT items ordered on a ‘mixed’ prescription and returning the prescription to the prescriber is not possible
- Provided the patient is not exempt from paying prescription charges for any other any reason, the patient should be asked to pay the required number of charges for all items dispensed (HRT and non-HRT items). For example, a prescription for Evorel Sequi patches and Naproxen 250mg tablets, the patient would pay a total of three charges (two charges for Evorel Sequi and one charge for Naproxen)
- Patient should be asked to mark the reverse of the EPS token with the correct number of charges paid for both HRT and non-HRT items dispensed. The patient should NOT select exemption reason ‘W’ for “HRT only prescription prepayment certificate”
- Dispensers should apply the ‘Paid’ EPS category code ‘0001’ on the electronic message
- If a patient does not have a valid HRT PPC at the point of dispensing and is unsure if they intend to purchase one, an FP57 Receipt and Refund form should be provided so that the patient can later claim a refund (within 3 months) for any listed HRT medicines covered by their backdated HRT PPC (or standard PPC). See FP57 receipt and refund form section for further information
- Note: If the patient is in possession of a valid HRT PPC at the time of dispensing, then only charges for the non-HRT items should be collected and the FP57 form should be completed at the same time for the HRT medicines. This will avoid the need for the pharmacy to collect and refund charges for any HRT medicines supplied at the same dispensing episode. As the prescription is marked as ‘Paid’, the NHSBSA will deduct charges for all items (HRT and non-HRT) on the prescription and the pharmacy can re-claim the charges for the HRT medicines dispensed by declaring the number of FP57 forms submitted and total value of charges to reclaim via the end of month FP34C submission form on MYS
The table below explains the different EPS category codes that can be selected when dispensing an electronic prescription for a patient with a ‘mixed’ prescription where both listed HRT medicines and non-HRT items are dispensed
Processing EPS prescriptions for other treatments that are not included in HRT medicines list
The steps below will guide you through the process to follow when presented with an electronic prescription for an item prescribed for the treatment of the menopause which is not an HRT medicine listed in Part XVI of the Drug Tariff
- The HRT PPC only covers applicable medicines in the HRT medicines list published in Part XVI of the Drug Tariff. A prescription for any other item which is not a listed HRT medicine (non-HRT item) should continue to be processed in the usual manner i.e. the patient would pay the applicable number of prescription charges unless they are exempt for other reasons
- If the patient is exempt from paying prescription charges, the patient should select the correct exempt reason on the reverse of the EPS token. The patient should NOT select exemption reason “HRT only prescription prepayment certificate” (EPS category code 0020) as the non-HRT item is not covered by the HRT PPC.
- Dispensers should apply the relevant EPS exemption category code on the electronic message, for example, “Medical exemption certificate” option (EPS category code ‘0006’ equivalent to the code ‘E’ on paper prescriptions and tokens)
- If the patient pays for the prescriptions, they should be asked to mark the reverse of the EPS token with the correct number of charges paid for the non-HRT medicines(s)
- Dispensers should apply the ‘Paid’ EPS category code ‘0001’ on the electronic message
The table below explains the different EPS category codes that can be selected when dispensing an electronic prescription for a non-listed medicine used for the treatment of menopause

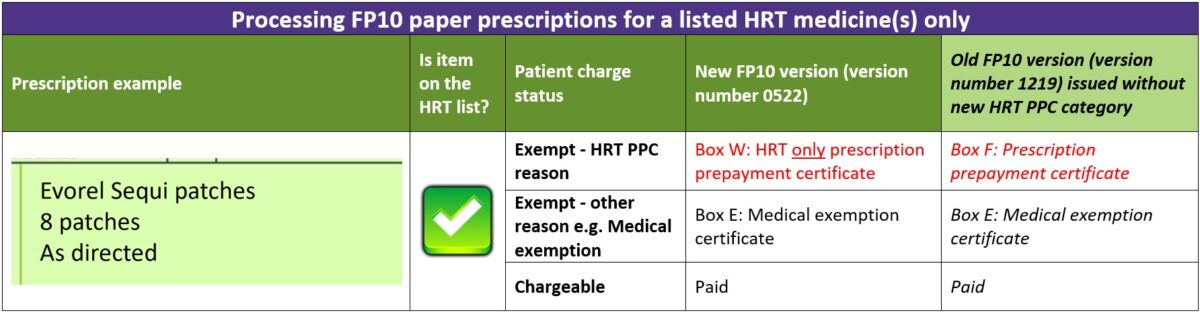
Listed HRT medicines only
The steps below will guide you through the process to follow when presented with an FP10 paper prescription for a listed HRT medicine only:
- Check if patient has a valid HRT PPC and that their prescribed HRT medicine(s) is covered by the HRT PPC
- If the patient holds a valid HRT PPC, advise the patient or their representative to select exemption reason ‘W’ for “HRT only prescription prepayment certificate” on the reverse of the paper FP10 prescription form.
- If presented with an older version of an FP10 form, advise the patient to select exemption reason ‘F’ for the standard “Prescription prepayment certificate”
The table below explains the different paid or exempt reasons for patients to select on the new or old FP10 form versions when dispensing a prescription for a listed HRT-only medicine to a patient
‘Mixed’ prescriptions with both listed HRT medicine(s) and non-HRT items
Processing FP10 paper prescriptions for ‘mixed’ items where there is an urgent need for either the listed HRT medicines or non-HRT items, or both. Also see:
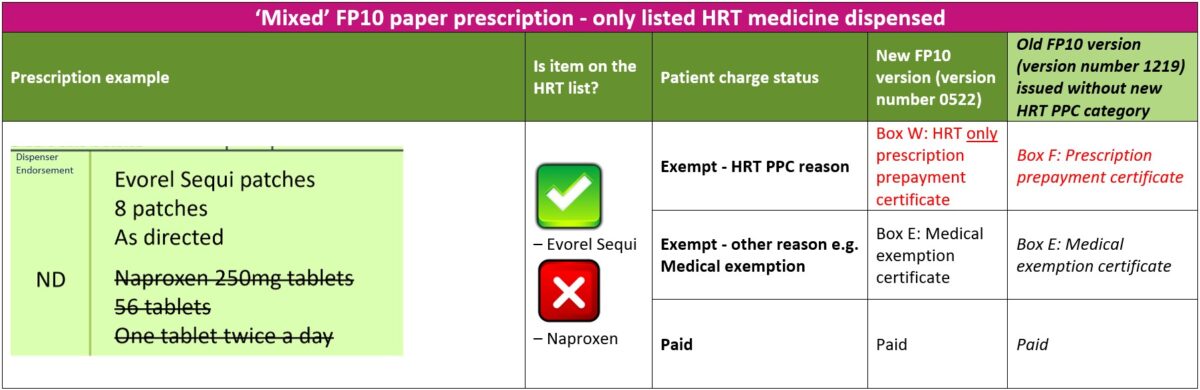
1. ‘Mixed’ prescriptions – only listed HRT medicine(s) dispensed
The steps below will guide you through the process to follow if a patient with a valid HRT PPC has an urgent need for the listed HRT medicines(s) ordered on a ‘mixed’ prescription
- Check if patient has a valid HRT PPC and that their prescribed HRT medicine(s) is covered by the HRT PPC
- Advise the patient to sign and date the reverse of the paper FP10 prescription form and tick exemption reason ‘W’ for “HRT only prescription prepayment certificate”. If presented with an older version of an FP10 form, advise the patient to select exemption reason ‘F’ for the standard “Prescription prepayment certificate”
- The non-HRT items should be crossed through and marked as ‘Not Dispensed’ or ‘ND’ in the dispenser endorsement section
- The patient should request the prescriber to re-issue a separate prescription for the non-HRT items not dispensed. Alternatively, the pharmacist can contact the prescriber on the patient’s behalf to obtain a new prescription for the non-HRT item(s) not dispensed
The table below explains the different paid or exempt reasons for patients to select on the new or old FP10 form versions when only listed HRT medicines are dispensed on a ‘mixed’ prescription
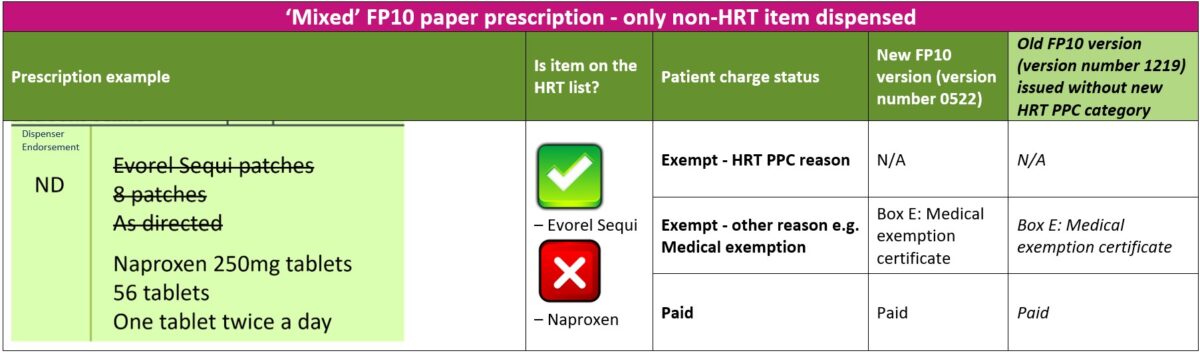
2. ‘Mixed’ prescriptions – only non-HRT medicine(s) dispensed
The steps below will guide you through the process to follow if a patient with a valid HRT PPC has an urgent need for the non-HRT items ordered on a ‘mixed’ prescription
- Check if patient has a valid HRT PPC and that their prescribed HRT medicine(s) is covered by the HRT PPC.
- Provided the patient is not exempt from paying prescription charges for any other any reason, the patient should be asked to pay the required number of charges for the urgent non-HRT items dispensed
- The patient should be asked to sign and date the reverse of the FP10 paper prescription form and enter the correct number of charges paid for the urgent non-HRT items dispensed. The patient should NOT select exemption reason ‘W’ for “HRT only prescription prepayment certificate”
- The listed HRT medicines should be crossed through and marked as ‘Not Dispensed’ or ‘ND’ in the dispenser endorsement section
- The patient should request the prescriber to re-issue a separate prescription for the listed HRT medicines not dispensed. Alternatively, the pharmacist can contact the prescriber on the patient’s behalf to obtain a new prescription for the listed HRT item(s) not dispensed
The table below explains the different paid or exempt reasons for patients to select on the new or old FP10 form versions when only non-HRT items are dispensed on a ‘mixed’ prescription
3. ‘Mixed’ prescriptions – both listed HRT medicine(s) and non-HRT items dispensed
The steps below will guide you through the process to follow if a patient has urgent need for both HRT and non-HRT items ordered on a ‘mixed’ FP10 paper prescription and returning the prescription to the prescriber is not possible
- Provided the patient is not exempt from paying prescription charges for any other any reason, the patient should be asked to pay the required number of charges for all items dispensed (HRT and non-HRT items). For example, a prescription for Evorel Sequi patches and Naproxen 250mg tablets, the patient would pay a total of three charges (two charges for Evorel Sequi and one charge for Naproxen)
- The patient should be asked to sign and date the reverse of the FP10 paper prescription form and enter the correct number of charges paid for both HRT and non-HRT items dispensed. The patient should NOT select exemption reason ‘W’ for “HRT only prescription prepayment certificate
- If a patient does not have a valid HRT PPC at the point of dispensing and is unsure if they intend to purchase one, an FP57 Receipt and Refund form should be provided so that the patient can later claim a refund (within 3 months) for any listed HRT medicines covered by their backdated HRT PPC (or standard PPC). See FP57 receipt and refund form section for further information
- Note: If the patient is in possession of a valid HRT PPC at the time of dispensing, then only charges for the non-HRT items should be collected and the FP57 form should be completed at the same time for the HRT medicines. This will avoid the need for the pharmacy to collect and refund charges for any HRT medicines supplied at the same dispensing episode. As the prescription is marked as ‘Paid’, the NHSBSA will deduct charges for all items (HRT and non-HRT) on the prescription and the pharmacy can re-claim the charges for the HRT medicines dispensed by declaring the number of FP57 forms submitted and total value of charges to reclaim via the end of month FP34C submission form on MYS
The table below explains the different paid or exempt reasons for patients to select on the new or old FP10 paper forms when both listed HRT medicines and non-HRT items are dispensed on a ‘mixed’ prescription
Q. How do deductions for HRT PPCs sold through pharmacies appear on the Schedule of Payments?
A. This will be displayed under the expense head of ‘HRT PPC’ on page 2 of the Schedule of Payments under ‘Details of Other Amounts Authorised’. Transactions for the existing standard PPC are displayed as ‘3 Mth Prepaid Cert’ and ’12 Mth Prepaid Cert’.
Guidance and FAQ briefing
The DHSC guidance has been published on the NHSBSA website:
Community Pharmacy England has published a briefing containing supplementary information and FAQs:
Community Pharmacy England HRT medicines poster (updated 1 June 2023)
NHSBSA has also published a communications toolkit which includes messaging for both primary care providers and people who may save money buying an HRT PPC:
HRT PPC communications toolkit
For more information on this topic please email comms.team@cpe.org.uk