Unlicensed specials and imports
Published on: 15th July 2013 | Updated on: 4th July 2024
Community Pharmacy England Briefing: Part VIIID – Specials with reimbursement prices relative to commonly identified pack sizes provides guidance on the new Part VIIID arrangements as FAQs covering the reasons for the introduction of a new section in the Drug Tariff for specials, Part VIIID price-setting processes as well as prescription endorsing and submission requirements.
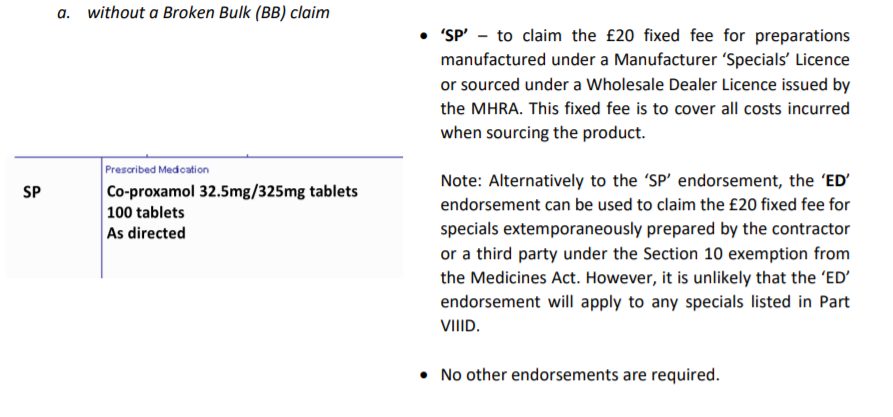
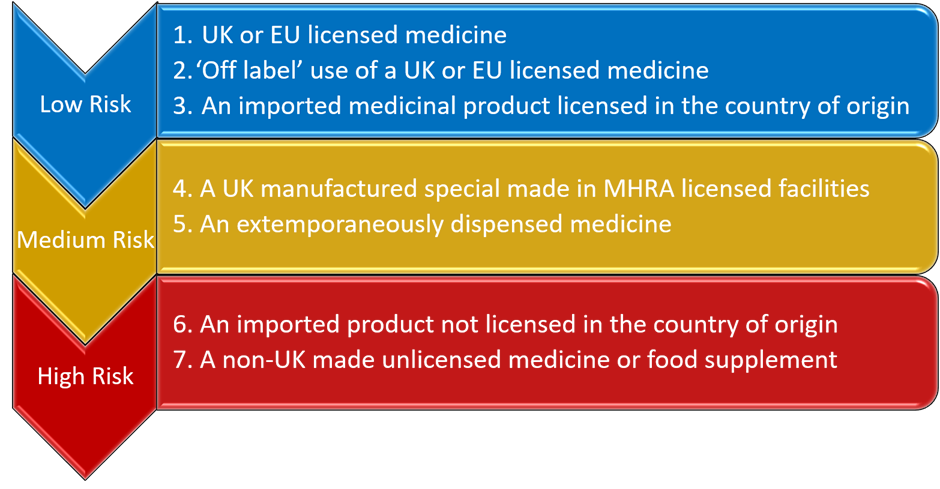
Pharmacy endorsing guidance for specials and imported unlicensed medicines factsheet. This factsheet outlines the differences in prescription endorsement requirements for both Drug Tariff-listed and non-Tariff specials. Specials “Specials are unlicensed medicinal products manufactured in the UK for human use which have been Imports “Imported products are unlicensed medicinal products sourced from outside the UK under an importers licence issued by the MHRA. These products have been specially sourced to meet a prescription ordered for individual patients without the need for the importer to hold a marketing authorisation for the medicinal product concerned.” Only specials that meet the minimum spend and volume requirements are included in the Drug Tariff. Parts VIIIB and VIIID of the Drug Tariff include high volume and high-cost unlicensed specials and imports, with set reimbursement prices based on volume or pack size. The prices are set by analysis of a selection of wholesaler and unlicensed specials manufacturer’s prices, with margin included for pharmacy purchase profit. The medicines list encompasses various formulations and preparations such as sugar free, preservative free, various flavours etc. Part VIIIB sets out payment arrangements for specials and imported unlicensed medicines (specials) to be paid with a price per unit above a minimum quantity. Please note: Broken Bulk CANNOT be claimed for any specials listed in Part VIIIB of the Tariff. The price listed in respect of a drug is the basic price on which payment will be calculated pursuant to Part I Clause 5B 1 for the dispensing of that drug. Drugs listed in this Part have a minimum quantity and price for that quantity and a price per subsequent ml/g/tab/cap unless in a special container. The Secretary of State determines the price based on information of manufactured specials or imports submitted by suppliers that hold a Manufacturer ‘Specials’ Licence or Wholesale Dealer Licence issued by the Medicine and Healthcare products Regulatory Agency (MHRA). Sales and volume data for Drug Tariff specials (Part VIIIB and Part VIIID) are obtained from suppliers under the Health Service Products (Provision and Disclosure of Information) Regulations 2018. This information is used to determine their reimbursement prices each quarter. Prices for Part VIIIB and Part VIIID specials will be updated quarterly (February, May, August and November). Part VIIID sets out payment arrangements for specials and imported unlicensed medicines (specials) to be paid relative to an identified pack size. The pack size listings are to support Broken Bulk claims which will be permitted for specials (except those classed as special containers) listed in Part VIIID. The price listed in respect of a drug is the basic price on which payment will be calculated pursuant to Part I Clause 5B 1 for the dispensing of that drug. The Secretary of State determines the price based on information of manufactured specials or imports submitted by suppliers that hold a Manufacturer ‘Specials’ Licence or Wholesale Dealer Licence issued by the Medicine and Healthcare products Regulatory Agency (MHRA). Sales and volume data for Drug Tariff specials (Part VIIIB and Part VIIID) are obtained from suppliers under the Health Service Products (Provision and Disclosure of Information) Regulations 2018. This information is used to determine their reimbursement prices each quarter. Prices for Part VIIIB and Part VIIID specials will be updated quarterly (February, May, August and November). In calculating Part VIIID reimbursement prices, for tablets and capsules, pack sizes up to and including 250-unit doses will be considered. Non-Tariff Specials are unlicensed medicines not listed in the Drug Tariff Parts VIIIB and VIIID these products can be sourced under a manufacturer’s specials/importers licence issued by the MHRA and reimbursement is based on endorsement. As contractors endorse the price after any discount is applied, and reimbursement is based on this price there is no need for the pharmacy to endorse ‘DNG’ on the prescription if no discount was obtained. Please note: Broken Bulk CANNOT be claimed for any non-Tariff special. For an unlicensed medicine which is not listed in Part VIIIB or Part VIIID of the Drug Tariff and has been manufactured under the Section 10 exemption from the Medicines Act 1968 (either by the contractor or a third party), endorse the names, quantities and cost of the ingredients used in preparing the product plus “ED” for costs incurred in dispensing a product prepared under the Section 10 exemption. Broken Bulk can be claimed on the ingredients used to make the product. Due to these separate arrangements for additional costs involved in sourcing an unlicensed special, Out of Pocket Expenses cannot be claimed against these items. The prescription endorsement requirements for specials and imported unlicensed medicines vary depending on whether a product is listed in the Drug Tariff (Part VIIIB or Part VIIID) but also how the special was sourced. Below are examples of the different prescription endorsement requirements for both Drug Tariff-listed and non-Tariff specials. The MHRA Guidance Note 14 – The supply of unlicensed medicinal products (“specials”) (Appendix 2) sets out the hierarchy of risk for the supply of unlicensed medicines that should be followed. A simplified diagram of the hierarchy is shown below: Broken Bulk Broken Bulk CAN be claimed on specials and imported unlicensed medicines listed in Part VIIID (unless the product is classed as a special container). To claim for Broken Bulk, the prescription must be endorsed with ‘BB’. Note: For Broken Bulk claims for Part VIIID specials, where the contractor endorses the prescription form ‘BB’ and regardless of any pack size endorsement, payment will be made for the nearest pack size or multiple of the pack size(s) listed in the Drug Tariff to the quantity prescribed. Price concessions can be granted for any product listed in Part VIIID. Please report any Part VIIID pricing issues to Community Pharmacy England here. Note: If a Part VIIID listed pack size is not available, a pack size concession can be granted by the Department. The updated pack size will be used for any Broken Bulk claims. Please report any pack size availability concerns to Community Pharmacy England. Certificate of Analysis (COA) / Certificate of Conformity (COC) For non-Drug Tariff specials, contractors must stamp, date, initial and endorse the COA / COC with the invoice price less discount and prescriber’s details and retain the COA / COC for 5 years. For non-Tariff imported unlicensed medicines, contractor shall make every reasonable effort to obtain a COA / COC for each imported product sourced and retain the COA/COC for 5 years. Where a COA / COC is available, contractors must stamp, date, initial and endorse the COA / COC with the invoice price less discount and prescriber’s details. If COC / COA is not available, the contractor must endorse required information on the invoice. The correct AMP product listing should be selected by prescribers when using their prescribing systems to ensure that the required product is supplied by the pharmacy so that contractors can be reimbursed accordingly. Click here for further information on dispensing incorrectly written prescriptions for specials. In EPS, a prescription for a special would read: It is important to note that if a prescriber adds free-typed supplementary information such as ‘Special’ or ‘Special Order’ into a different EPS field for example, as part of the dosage instructions or additional information field, this will not be taken into account for reimbursement purposes as this information is not captured by the NHS Business Services Authority (NHSBSA) during processing. For further information on the inclusion of additional product information within the dosage area field see cpe.org.uk/dosearea. Note: GP system suppliers currently undertake to map no less than 95% of the most frequently prescribed drugs in primary care. Prescribing system suppliers should continue to map to greater numbers of dm+d listings so that more prescriptions can be sent via EPS instead of only via paper prescriptions. More about dm+d mapping at our dm+d webpage. FP10 paper prescriptions for specials or imported unlicensed medicines should continue to be placed in the relevant red separator as part of the end of month submission process. There are no virtual red separators for EPS prescriptions. Prescriptions that would ordinarily be placed in a red separator are processed accordingly, when submitted electronically. The “SP” and “ED” fees paid for the dispensing of unlicensed medicines will be displayed in the Prescription Fees section on page 1 of the Schedule of Payments. The total value of these fees paid to the contractor for the month in question will be displayed under “Additional fees – 2A unlicensed medicines”.
specially prepared to meet a prescription ordered for individual patients without the need for the
manufacturer to hold a marketing authorisation for the medicinal product concerned.”Specials listed in Part VIIIB

Specials listed in Part VIIID

Specials not listed in the Drug Tariff


Drug Tariff listed specials
Non-Drug Tariff specials
Part VIIIB
Part VIIID
Pricing arrangements
Specials and imported unlicensed medicines listed in Part VIIIB are currently restricted to manufactured non-solid dosage forms (for example liquids, creams, ointments and lotions etc.) which, except for products classed as special containers, are reimbursed based on a price per unit above a minimum quantity.
Products listed in Part VIIID will be specials and imported unlicensed medicines (mainly unlicensed tablets and capsules) with reimbursement prices calculated relative to commonly identified pack sizes.
All non-Drug Tariff specials are reimbursed based on endorsement of the invoice price (less any discount and rebate) of the pack size used.
Broken Bulk CANNOT be claimed on specials and imported unlicensed medicines listed in Part VIIIB.
Broken Bulk CANNOT be claimed on any non-Drug Tariff unlicensed specials and import unless the claim is for the individual ingredients used to prepare an extemporaneously dispensed specials under the manufacturing part of the Section 10 exemption from the Medicines Act 1968).
Discount deduction
All Part VIIIB specials and imported unlicensed medicines are subject to the discount deduction scale unless the product meets any of the Group Items Discount Not Deducted (DND) criteria for example a Schedule 2 or 3 Controlled Drug or require cold storage.
All Part VIIID specials and imported unlicensed medicines are subject to the discount deduction scale unless the product meets any of the Group Items Discount Not Deducted (DND) criteria for example, a Schedule 2 or 3 Controlled Drug or requires cold storage.
As Group Items, all specials and imported unlicensed medicines not listed in the Drug Tariff are automatically exempt from any discount deduction i.e. all non-Drug Tariff specials are automatically granted DND status
Price concessions
Price concessions can be granted for any product listed in Part VIIIB. Please report any Part VIIIB pricing issues to Community Pharmacy England here.
N/A
No COA / COC needed
No COA / COC needed
Record keeping requirements
When supplying an unlicensed special or imported unlicensed medicine to a patient, in all instances the pharmacy is required by the MHRA to keep a record of the following information for 5 years:
FAQs
Where there is an indication that the Part VIIIB item has been supplied, you will be reimbursed the Part VIIIB price. If however there is an endorsement of the specific manufacturer requested and other information such as price is also clearly endorsed (see our Dispensing Factsheet – Unlicensed Specials and Imports in the Resources section below) then you will be paid as endorsed.
If a prescriber orders a Part VIIID special by brand or includes details of a specific supplier to obtain the special from, contractors will be reimbursed based on their endorsement (i.e., as a non-Tariff special). For specials ordered via EPS, the prescriber will need to select the correct product description (AMP) if available on prescribing systems or, consider providing a replacement paper FP10 prescription from with the brand or supplier specified as part of the drug name to ensure that it can be accurately priced by the NHSBSA.
As it would not be practical to list every available pack size of a special listed in Part VIIID, only the most common identified pack size(s) based on the latest two quarters’ specials data submitted by suppliers to DHSC are listed. For some Part VIIID specials, more than one pack size may be listed if this is supported by the available data. The pack size listing is intended to support Broken Bulk claims which are permitted for specials listed in Part VIIID.
Unless Broken Bulk is claimed, contractors are reimbursed for the exact quantity prescribed (unless less dispensed). Using the example of Mepacrine 100mg tablets, the reimbursement price in the April 2022 Drug Tariff is listed at £223.09 for a pack of 50. If a prescription is received for 8 tablets and Broken Bulk was not claimed, reimbursement would be calculated pro-rata based on the price of the Tariff listed pack size. Payment is calculated pro rata using the basic price of £223.09, so for 8 tablets reimbursement is calculated as follows:
Calculate the price per tablet: £223.09/50 = £4.46
Eight tablets are required so £4.46 x 8 = £35.70
The basic price for 8 Mepacrine 100mg tablets is £35.70
If Broken Bulk is claimed, contractors are reimbursed for the nearest pack size or multiple of Drug Tariff listed pack size(s) sufficient to supply the quantity prescribed, example below:
Mepacrine 100mg tablets
Quantity prescribed: 8 tabs
Pack size(s) listed in Part VIIID: 50
Contractors will be reimbursed for pack size of 50, as this is the nearest Drug Tariff listed pack size to quantity prescribed (8).
The reimbursement price for 8 Mepacrine 100mg tablets with a broken bulk claim is £223.09
Yes, price concessions can be granted for any specials listed in the Drug Tariff. Any pricing issues affecting Part VIII drugs (including specials) can be reported to Community Pharmacy England for further investigation. Contractors should report pricing issues to Community Pharmacy England here.
No, Broken Bulk claims are ONLY permitted for specials listed in Part VIIID. Note: Broken Bulk CANNOT be claimed for any Part VIIIB special. Also, Broken Bulk CANNOT be claimed for any non-Tariff special unless the claim is for the individual ingredients used to prepare an extemporaneously dispensed specials under the manufacturing part of the Section 10 exemption from the Medicines Act 1968).
When Broken Bulk is claimed for a Part VIIID special, contractors are reimbursed for the nearest pack size or multiple of Drug Tariff listed pack size(s) sufficient to supply the quantity prescribed. Broken bulk claims are paid against the pack size(s) listed in the Drug Tariff. If a contractor claimed BB, regardless of pack size used, contractors would be paid for the following pack sizes in the examples given below.
Nadolol 40mg tablets
Quantity prescribed: 90 tabs
Pack size(s) listed in Part VIIID: 28 and 100
Contractors will be reimbursed for pack size of 100, as this is the nearest Drug Tariff listed pack size to quantity prescribed (90).
Melatonin 10mg capsules
Quantity prescribed: 168
Pack size(s) listed in Part VIIID: 30 and 60
Contractors will be reimbursed for 3 multiples of 60 as this is closest to the quantity prescribed (168).
Metolazone 2.5mg tablets
Quantity prescribed: 14
Pack sizes listed in Part VIIID: 4 and 100
Contractors will be reimbursed for 4 multiples of 4 as this is closest to the quantity prescribed (14).
Broken bulk claims are allowed on products in Part VIIID if it is unlikely that the balanced will be used within the following six months. Subsequent prescriptions for the same Part VIIID special, received during the next six months, will be deemed to have been supplied from the remainder and no further payment will be made to drug costs other than fees and consumables and container allowances until that remainder has been or is deemed to have been used up. A new Broken Bulk claim for the Part VIIID special can only be submitted once the remaining balance is used up or after six months of the original claim, whichever is earlier.
Yes, all FP10 paper prescription forms for Drug Tariff-listed and non-Tariff specials are required to be placed in the relevant red separator for end of month submission.
The Drug Tariff no longer has a requirement for contractors to submit copies of the Certificate of Analysis (COA) or Certificate of Conformity (COC) to the local NHS England and NHS Improvement (NHSE&I) team of the prescriber after dispensing unlicensed specials or imports not listed in the Drug Tariff.
As contractors are still required to keep the necessary records of unlicensed specials or imports they dispense for a period of five years, any COAs and COCs obtained should be retained by the pharmacy for this purpose.
If the value of the Part VIIID special dispensed exceeds £100 this will be detailed on the Schedule of Payments under section titled Summary of expensive items. Payment information can also be accessed on the Prescription Item Report (PIR) which is a monthly data report containing item-level payment information showing the reimbursement and remuneration calculated by the NHSBSA for each item submitted for payment. For more information see our Factsheet: How to access your Prescription Item Reports.
Related resources
Dispensing Factsheet: Prescription Endorsement
Dispensing Factsheet – Unlicensed specials and imports
Are you dispensing incorrectly written prescriptions for specials
MHRA Guidance Note 14 – The supply of unlicensed medicinal products
GPhC guidance on preparing unlicensed medicines
Royal College of Ophthalmologists specials guidance
British Association of Dermatology specials guidance
For more information on this topic please email comms.team@cpe.org.uk