Broken bulk
Published on: 3rd May 2013 | Updated on: 6th March 2024
The broken bulk (‘BB’) endorsement enables pharmacy teams to claim payment for a complete pack of an eligible product where only a part-pack has been dispensed. For a claim to be accepted, contractors must dispense from the pack or combination of packs that minimises the residual balance. Contractors should only claim ‘BB’ where it is unlikely that they will be able to dispense the remainder within the following six months (see ‘Subsequent supplies within six months’ below for more information).
Eligibility
The products on which ‘BB’ can be claimed are as follows:
 |
 |
|
|
* Unless claiming for ingredients used to prepare an unlicensed special extemporaneously dispensed.
Endorsements
To ensure payment, contractors must endorse:
- ‘BB’ ; and
- The quantity dispensed over pack size used (see example below).
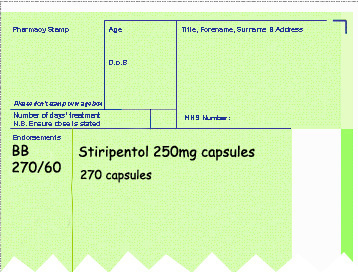
Endorsement for Part VIIID products

Sorting and submission
Prescription forms endorsed with a ‘BB’ expense claim need to be separated and placed in the red separator before submission.
Subsequent supplies within six months
When a ‘BB’ claim is accepted, contractors are reimbursed for the complete pack dispensed. Subsequent prescriptions for the same medicine received during the next six months do not need an endorsement of ‘BB’; this is because it will be deemed that supply was made using the residual balance. During this time, no further payment will be made other than fees and consumable allowances until the remainder has been used up.
Schedule of Payments
The FP34 Schedule of Payments includes a summary of expensive items. In this section you will find a breakdown of Expensive Items where the number and value of items are equal to or over £100 before any adjustments for ‘BB’.
For more information see our page Understanding your FP34 Schedule of Payment.
Re-charging
The cost of the quantity prescribed for the drug where broken bulk is claimed is charged back to the prescribers CCG’s budget. The difference in cost between the prescribed quantity and the dispensed quantity for a broken bulk claim is centrally retained and fairly shared across all Primary Care Organisations who issue prescriptions under Primary Care Services.
FAQs
‘BB’ cannot be claimed on unlicensed specials manufactured or supplied by a company operating under an MHRA specials licence. However, it should be noted that Zopiclone 7.5mg/5ml oral suspension is listed in Part VIIIB of the Drug Tariff with a minimum reimbursement price set against a pack size of 100ml. As the minimum volume listed in the Drug Tariff is 100ml reimbursement would be for 100ml where less was prescribed.
Please note if a product has been extemporaneously dispensed, a claim for ‘BB’ is allowed on the ingredients used to make it. This is to cover times where you may not have the opportunity to use the remainder of the open container used in preparing the special.
No, you cannot claim ‘BB’ on a prescription for Sando-K effervescent tablets. This is because Sando-K effervescent tablets have special container status and items with special container status are not eligible for a claim of ‘BB’.
In this scenario, the special container status is on the sub-pack of 20 meaning that reimbursement on a prescription calling for Sando-K effervescent tablets x 20 tablets would be for 20 tablets.
For information on the special container rules see our page Special containers (including dispensing of oral liquid antibiotics requiring reconstitution).
Your pharmacy system should enable you to make a ‘BB’ claim through manual endorsement against the item during the dispensing process. If you are unclear on how to do this, refer to your pharmacy system manual or speak with your pharmacy system supplier for guidance.
Related resources
Sorting your prescriptions prior to submission
Understanding your FP34 Schedule of Payment
For more information on this topic please email comms.team@cpe.org.uk









