Hub & Spoke
Published on: 21st May 2024 | Updated on: 19th December 2025
|
Status: Came into force 1 October 2025. Overview: Dispensing processes can be shared between different retail pharmacy businesses. (Same legal entity hub and spoke arrangements are not affected by this change.) Amendments have been made to the Human Medicines Regulations 2012 (HMR) and the NHS (Pharmaceutical and Local Pharmaceutical Services) Regulations. Full information is outlined in our briefing: Briefing 020/25: Hub and Spoke dispensing between different retail pharmacy businesses |
Hub and spoke dispensing Terms of Service
The new Terms of Service provide that contractors may not subcontract ‘core dispensing activities’ (broadly the assembly or part assembly of any prescription item, which includes labelling and bagging) unless certain conditions are met. Briefly, these are as follows:
Key requirements for spoke pharmacies
An NHS pharmacy (spoke) may subcontract core dispensing activities to a hub pharmacy, as part of hub and spoke arrangements, only if certain requirements are met. The contractor (spoke) must, for example:
- Take reasonable steps to ensure that the owner of the hub pharmacy is a fit and proper person to carry out the core dispensing functions on their behalf.
- Give notice to their integrated care board (ICB) of the hub and spoke arrangements (using the NHS England-approved notification form), generally not less than 28 days before the hub and spoke arrangements are intended to commence.
- Ensure the core dispensing functions are to be sub-contracted under written hub and spoke arrangements (with the hub pharmacy owner) that include specific provisions as detailed in the NHS England-approved notification form and confirm those in the notification to the ICB.
- Display a notice for patients, conspicuously, at the registered pharmacy (or on the pharmacy website for online dispensing, e.g. DSP pharmacies), which contains the name of the hub pharmacy owner and the address of the hub and a brief statement of the general effect of the hub and spoke arrangements.
- Comply with the requirements of the information gateway by, for example, displaying an appropriate notice to patients at the pharmacy and ensuring all staff maintain the confidentiality of the patient information. The pharmacy’s privacy notice should also be updated.
- Ensure the dispensed medicine is labelled with the spoke’s name and address, and the date on which the hub assembled, or part assembled the medicine (as well as the usual information required on a dispensing label).
- Ensure that if a hub pharmacist changes a supply (as the spoke pharmacist might), the final decision on the supply is made by the supervising spoke pharmacist, even if this confirms the decision made by the hub pharmacist. (An example of this is using the original pack dispensing +/-10% provision.)
- Give notice in writing to the ICB of any suspension or permanent discontinuation of hub and spoke arrangements, before this occurs or as soon as reasonably practicable afterwards.
A link to the NHS England-approved notification form is available here.
There is provision for ICBs to raise objections to proposed hub and spoke arrangements by issuing a relevant notice, for dispute resolution with the contractor, and, at the contractor’s request, the involvement of the LPC. If the ICB issues a notice of objection, the contractor must not commence the hub and spoke arrangements until or unless that notice is withdrawn.
Timescales
Since the earliest an NHS spoke pharmacy will be able to notify their ICB is the date on which the HMR amendments came into force (1 October 2025), the use of hub and spoke dispensing would only be able to start at the earliest, on 29 October 2025.
The new hub and spoke Terms of Service (The National Health Service (Pharmaceutical and Local Pharmaceutical Services) (Miscellaneous Amendments) Regulations 2025) came into force on 1 October 2025, the same day as the Human Medicines Regulations 2012 (HMRs) hub and spoke changes (as detailed below). They complement each other and apply to owners of NHS spoke pharmacies/contractors when the hub pharmacy is owned by a different legal entity. To carry out such hub and spoke dispensing, pharmacy owners must comply with the requirements of: (Hub pharmacy owners must comply with the HMRs and may be impacted by contractors complying with their Terms of Service.) Additional provisions/notes There is some overlap between the HMR and Terms of Service requirements because NHS England is seeking confirmation that certain actions required by the HMRs have been completed. There is recognition in the new Terms of Service that NHS hub and spoke dispensing may include non-medicines, such as appliances, and may use, for example, Serious Shortage Protocols, and appropriate provision for this. Any temporary discontinuation of hub and spoke arrangements that amounts to a suspension or permanent discontinuation must be notified to the ICB, either before or as soon as is reasonably practicable after it occurs. The Government confirmed that it is introducing Hub and Spoke dispensing Model 1 and changes to the Human Medicines Regulations 2012 have been laid The Human Medicines (Amendments Relating to Hub and Spoke Dispensing etc.) Regulations 2025. Key points of the HMR amendments in brief are: Community Pharmacy England is discussing with the Department of Health and Social Care (DHSC) associated amendments to the NHS (Pharmaceutical and Local Pharmaceutical Services) Regulations (PLPS regulations) to permit the NHS Spoke pharmacy to subcontract part of the NHS Essential Dispensing Service to the hub. These amendments are likely to include a requirement that NHS spoke pharmacies must notify their Integrated Care Board (ICB) 28 days in advance of commencing hub and spoke dispensing (for NHS prescriptions).
Hub and spoke dispensing – essentially sharing the dispensing process between two pharmacies – is currently permitted within the same retail pharmacy business (i.e. the same legal entity), but planned regulatory changes will permit it between different retail pharmacy businesses (i.e. different legal entities).
In 2022, DHSC consulted for a second time on Hub and Spoke dispensing between community pharmacy businesses. They proposed two models:
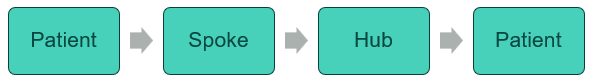
- Model 1, where the medicines are returned assembled from the hub to the spoke pharmacy for supply to the patient (patient – spoke – hub – spoke – patient)
- Model 2, where the hub pharmacy supplies medicines directly to the patient (patient – spoke – hub – patient)
We responded to the consultation (see our response here) and have since continued to reinforce our view with DHSC that only Model 1 should be introduced.
The full debate on hub and spoke can be found on the Hansard – UK Parliament website: Community Pharmacy – Hansard – UK Parliament
Proposed Models
The Department of Health and Social Care (DHSC) issued a consultation (Government response to the consultation on hub and spoke dispensing) on hub and spoke dispensing, in 2022. The consultation proposed making legislative changes to allow the operation of hub and spoke dispensing across different legal entities, and proposes to introduce two different models for this. Model 1 would involve patients only interacting with the spoke pharmacy, whilst Model 2 would permit the hub to supply medicines directly to the patient on behalf of the spoke.
Model 1

Model 2
Gordon Hockey, Director, Legal at Community Pharmacy England, said:
“Community Pharmacy England broadly supports the introduction of changes that would allow hub and spoke dispensing to occur between different legal entities, but they need to be introduced in a way that is safe for patients and does not lead to a proliferation of hubs that can dispense directly to patients, which would undermine market entry controls. Without controls on hubs, the community pharmacy market could be disrupted.
“We are also concerned that while hub and spoke dispensing may potentially release capacity in community pharmacies, there is no evidence that it will lead to financial savings for the sector. In addition, any activity saving will come at a cost, and given the critical state of pharmacy finances, pharmacy owners may not be able to invest in this at the moment.
“The previous Government published its intent to progress the changes it had consulted on, but this work was paused when the General Election was called. The Department of Health and Social Care is now in the process of briefing new ministers across all policy areas, including hub and spoke. Officials have indicated this process may take some time and the sector will be given more clarity on timescales as soon as practicable.
“In the meantime, the Department has confirmed that it will continue to discuss the relevant issues with Community Pharmacy England. This includes patient safety issues and the potential proliferation of hubs, which are primarily issues with the Department’s Model 2 (patient – spoke – hub – patient) where supply of the dispensed medicine is from the hub direct to the patient.”
Q. Why is there an overlap between the PLPS and HMR regulations?
There is an overlap between the PLPS and HMR regulations because NHS England is seeking confirmation that certain actions required by the HMRs have been completed.
Q. Can all prescription items be dispensed using hub and spoke arrangements?
Yes. There is recognition in the new PLPS regulations that NHS hub and spoke dispensing may include all prescription items, for example, non-medicines, such as appliances, and include certain supply methods in addition to prescriptions, for example, Serious Shortage Protocols. To
accommodate this, the PLPS regulations provide an information gateway for these additions, to ensure the use of patient information is permitted for all items supplied using hub and spoke arrangements (provided the relevant conditions are met, such as a notice to patients).
Q. Why do these requirements only apply to hub and spoke arrangements between different retail pharmacy businesses, not intracompany arrangements?
There are several reasons:
First, section 10 of the Medicines Act 1968 has long permitted hub and spoke dispensing within the same retail pharmacy business (intra-company).
Second, intra-company hub and spoke arrangements do not involve subcontracting of any part of the Essential dispensing service. This is carried out by the listed contractor, and supplies to patients are made from the contractor.
Third, there are additional issues and risks associated with (and corresponding measures and controls required for) a joint dispensing process between different legal entities, which are addressed in the new HMR and PLPS regulations, and the GPhC guidance on hub and spoke
dispensing.
Q. Isn’t the hub supply of assembled medicines to a spoke a wholesale transaction?
The HMRs provide that the supply of (an assembled) medicine from a hub to a spoke, as part of hub and spoke arrangements set out in the HMRs (in effect as part of a shared process for dispensing patients’ prescribed medicines), is not a wholesale transaction but a deemed retail
transaction.
Q. How do I notify my ICB of a proposed hub and spoke arrangement?
You must notify your ICB using the NHS England-approved notification form. NHS England states that currently:
‘… notifications of a hub and spoke arrangement may be made to the ICB pharmacy contract team by email using the attached form. However, this form will be made available in the NHS Business Service Authority (NHSBSA) Manage Your Service MYS portal for these notifications
from early 2026. Once operational, wherever possible, the notifications made in relation to a hub and spoke arrangement (starting, ending or being suspended) must be made via the NHSBSA MYS portal. Only on the occasions that this system is unavailable or contractors do not have an MYS sign-on will notification be accepted by email to the email address of their ICB pharmacy contract team as detailed on the pharmacy contract teams web page.’
Q. Can a spoke enter into more than one hub and spoke arrangement?
Yes, a spoke can enter into hub and spoke arrangements with more than one hub. Each arrangement must be notified to the ICB.
Q. What subcontracting of core dispensing functions is allowed in addition to (valid) hub and spoke arrangements?
A contractor may subcontract core dispensing functions to:
- A locum (or provider of locums) (including through a subsidiary company (undertaking) of the contractor (or subsidiary of a parent company where the contractor is also a subsidiary)), and
- As part of temporary (management) arrangements related to the purchase of a pharmacy, where one retail pharmacy business is carrying on the business (on behalf of) the other retail pharmacy business at the listed pharmacy premises.
Q. May dispensing doctors have hub and spoke arrangements for the supply of medicines on prescription?
Yes. The HMRs provide that dispensing doctors may be spokes in hub and spoke arrangements for the supply of medicines on prescription. The hub must be a registered pharmacy.
Q. Can schedules 1 to 3 controlled drugs be dispensed under a hub and spoke arrangement between two different legal entities?
No, schedules 1 to 3 controlled drugs must not be assembled or dispensed through hub and spoke arrangements between two different legal entity dispensing businesses as this would not comply with the provisions of the Misuse of Drugs Regulations 2001. All types of orders for these schedules of controlled drugs therefore must be assembled and dispensed at the spoke pharmacy.
Q. Can a private, non-NHS hub pharmacy enter into hub and spoke arrangements with an NHS spoke pharmacy?
Yes, non-NHS pharmacies can enter into hub and spoke arrangements as the hub pharmacy.
Q. How will hub pharmacies claim the cost of dispensed items from the spoke pharmacy?
This is a matter of contract between the hub pharmacy and the spoke pharmacy. The Hub pharmacy will not be submitting prescriptions to the NHS Business Services Authority (NHSBSA) that have been dispensed under hub and spoke arrangements. The spoke pharmacy will claim for the dispensing that is done by the hub. Therefore, a private contract between the hub and spoke pharmacies will need to be agreed upon as part of the hub and spoke arrangements to address costs.
Q. Can schedule 2 controlled drugs be processed as part of the hub and spoke arrangements between different legal entities?
No, schedules 1 to 3 controlled drugs must not be assembled or dispensed through hub and spoke arrangements between different legal entity dispensing businesses, as this would not comply with the provisions of the Misuse of Drugs Regulations 2001. All types of prescriptions for these schedules of controlled drugs, therefore, must be assembled and dispensed at the spoke pharmacy.
Q. Can hub and spoke arrangements be between a pharmacy and a dispensing doctor’s practice?
Yes, however, a dispensing doctor’s practice cannot be a hub pharmacy. Therefore, a dispensing doctor’s practice can enter into hub and spoke arrangements as the spoke pharmacy. Any prescriptions processed under these arrangements would still need to be supplied to the patient by the dispensing doctor’s practice (spoke).
Q. Does the spoke pharmacy need to relabel the medicines once they are received from the hub?
No, the hub will label the medicines with the date of preparation in the hub. However, the label must contain the name and address of the spoke.
Q. Does the spoke pharmacy determine what is included on the medicine dispensing label?
Yes, the spoke pharmacy will determine what is included on the medicine dispensing label. The hub pharmacy will be sent prescription information from the spoke pharmacy.
Q. Can the hub pharmacy be a distance selling premises (DSP) pharmacy?
Yes, DSP pharmacies can be hub pharmacies as part of hub and spoke arrangements. However, the supply to the patient must still be made by the spoke pharmacy and not directly from the hub pharmacy (DSP), as a DSP would normally process prescriptions.
Q. Can prescriptions that require delivery be delivered to the patient directly from the hub pharmacy?
No, the supply to the patient must be made from the spoke pharmacy. Therefore, prescriptions must be returned to the spoke pharmacy before a delivery is made to the patient.
Q. Can a spoke pharmacy enter into multiple hub and spoke arrangements?
Yes. The spoke pharmacy will need to conspicuously display notices for each hub and spoke arrangement it is part of. The spoke will also need to notify the ICB of those arrangements.
Q. Are all NHS pharmacies expected to enter into hub and spoke arrangements?
No. The change to the legislation is enabling. This means that pharmacies can choose to use the provision or not.
Hub and spoke dispensing is already permitted between community pharmacies owned by the same legal entity (the same retail pharmacy business). These changes will permit different pharmacy owners (different retail pharmacy owners) to carry out hub and spoke dispensing if they comply with the relevant requirements.
Hub and Spoke is one of several important regulatory changes currently underway to help make dispensing more efficient and support capacity for the provision of clinical services. To learn more about these and Hub and Spoke, read our new guide: New Pharmacy Regs: What you need to know
Further information
Community Pharmacy England (formerly PSNC) submits response to Hub and Spoke consultation
For more information on this topic please email regulations.team@cpe.org.uk













