Referred back and disallowed items
Published on: 8th February 2021 | Updated on: 8th December 2025
Community pharmacies process and dispense a wide range of products ordered by a variety of healthcare professionals. Occasionally, the NHS Business Services Authority (NHSBSA) may return prescription items to pharmacy teams, either referred back or disallowed. Referred back items can result in delayed payments, whereas no payments are made for any disallowed items.
This page explains what referred back items (or unpaid items) and disallowed items are, outlines the reasons why the NHSBSA refer back or disallow certain items, and highlights the checks that can be implemented during the dispensing process to help minimise the risk of receiving referred back or disallowed items.
In 2024/25, around 1 million prescription items were referred back to pharmacy owners by the NHSBSA due to missing or insufficient endorsed information. This works out to a total of 85,000 items referred back to community pharmacies each month.
When the NHS Business Services Authority (NHSBSA) cannot process a prescription item due to insufficient endorsed information, it is returned to the pharmacy team, and this is known as a referred back item. Referred back items are returned to the pharmacy via the MYS portal for the missing information to be added so that it can be correctly reimbursed. Part II Clause 9F of the Drug Tariff states ‘where insufficient information is available to enable the Pricing Authority to process the prescription, including where it was submitted for a SSP payment, the form (or a copy of the original form) shall be returned to the contractor who shall endorse the prescription form (or copy) with the information requested. Returned prescriptions shall be priced using the Drug Tariff relevant to the month in which the prescription form (or copy) is returned to the Pricing Authority.’ The most common reason why an item is returned is due to an incomplete endorsement, where one or more of the following is missing: An item could also be returned if too much information is included in the endorsement, for example more than one manufacturer endorsed.
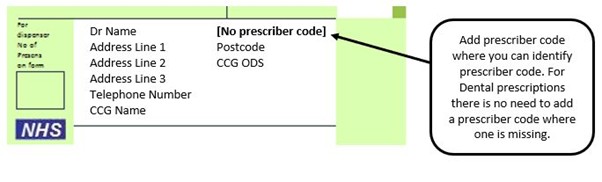
The NHSBSA apply a coding system to identify up to 19 possible reasons for items being referred back to the pharmacy for further information. Each referred back item is assigned a referred back code to indicate the referred back reason. The table below shows referred back codes used for community pharmacy: This reason code is usually used where the pack size and price have been endorsed on initial submission, but the supplier or manufacturer or brand name was not stated. This reason code is often used where the supplier, manufacturer or brand name has been endorsed on initial submission, but the pack size and price were not stated. The presentation details for the item are not included in the prescribed order or in the endorsement. You need to endorse the presentation dispensed. The strength details for the item are not included in the prescribed order or in the endorsement. You need to endorse the strength dispensed. The full product name is not included in the prescribed order or in the endorsement. You need to endorse the exact product dispensed. This reason code is often used where a brand name indicates a range of products rather than a single product. You need to endorse which product from the range was dispensed. The size details for the item are not included in the prescribed order or in the endorsement. You need to endorse the size. The quantity details for the item are not included in the prescribed order or in the endorsement. You need to endorse the quantity dispensed. This reason code is often used when appliances are ordered as a number of ‘boxes’. You need to endorse the total quantity dispensed. The type or brand of appliance dispensed is not included in the prescribed order or in the endorsement. You need to endorse the type or brand dispensed. This reason code is often used when an appliance item is ordered generically but is only listed as branded items in Part IX of the Drug Tariff. You need to endorse the brand supplied. The catalogue number is not included in the prescribed order or in the endorsement. You need to endorse the catalogue number. The catalogue number, as listed in the Drug Tariff may be required to identify the exact product dispensed for catheters, incontinence appliances, stoma appliances or lymphoedema garments. Each account type is restricted in which form types can be submitted within it. Form types which are not allowed in a particular account type are referred back so they can be resubmitted in the correct account type. This reason code is often used for private Controlled Drug forms and Out of Hours Non FP10 Supply forms which are submitted in pharmacy contractor NHS accounts. The size of the vial, ampoule or cartridge dispensed is not included in the prescribed order or in the endorsement. You need to endorse the size dispensed. This reason code is used where there are numerous sizes of vial, ampoule or cartridge available for the prescribed product and the size dispensed is not indicated. The strength, quantity or presentation of the Controlled Drug dispensed is not included in the prescribed order. This information needs to be included in accordance with The Misuse of Drugs Regulations 2001. The prescribed order and endorsement do not contain enough information for NHSBSA to determine the item intended. You need to endorse the exact item dispensed so reimbursement can be made for the item. You may also need to endorse any other relevant information. This could include the supplier, invoice price, pack size, strength, quantity or presentation. This reason code is often used if there are contradicting printed and handwritten endorsements against an item or if handwritten prescribed orders or endorsements are illegible.
Referred back (RB) code
Referred back reason
RB1A
For prescribed items not listed in Part VIIIA of the Drug Tariff all of the following endorsements are required:
RB1B
For prescribed items not listed in Part VIIIA of the Drug Tariff all of the following endorsements are required:
RB1C
For prescribed items not listed in Part VIIIA of the Drug Tariff all of the following endorsements are required:
RB1D
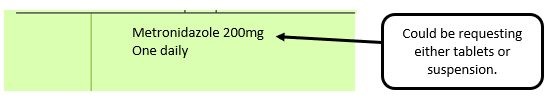
For prescribed liquid items that are unlicensed medicines without the pharmaceutical form specified or defined as liquid. Either solution or suspension needs to be stated.
RB1E
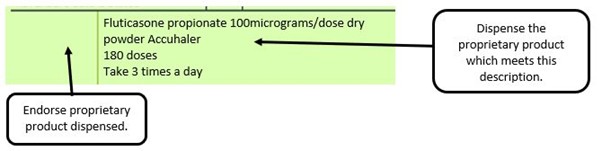
For prescribed items available from more than one manufacturer with the same brand name the manufacturer of the item supplied needs to be endorsed.
RB2A
For prescribed items missing the presentation, strength or quantity, these must be endorsed.
RB2B
RB2C
RB2D
The quantity details for the item are not included in the prescribed order or in the endorsement. You need to endorse the quantity dispensed.
RB2E
RB3A
The size length or width, quantity, type or manufacturer’s catalogue number details for the item are not included in the prescribed order or in the endorsement.
RB3B
RB3C
RB3D
RB3E
RB4
RB5
RB6
RB7
Pharmacy owners will receive an email to their pharmacy NHSmail account if any new unpaid items have been returned by the NHSBSA for further information. All returned items for both electronic and paper prescriptions can be viewed under the tab titled ‘Unpaid items’ on the Manage Your Service (MYS) portal landing page. All referred items must be completed and submitted via MYS. Under the Unpaid Items tab, the ‘missing information’ section will show details of all returned prescription items along with the month in which the item was originally submitted for payment, the submission deadline and the name of the unpaid item. To view the information required by the NHSBSA, pharmacy teams should select ‘add information.’ Each returned item will display the product details and specify what additional information the NHSBSA needs to process the payment. The image below shows how referred back items appear in MYS. Once the required additional information has been added to the relevant sections within MYS, pharmacy teams can submit the referred back item electronically to the NHSBSA from MYS for processing. Managing referred back prescriptions through MYS allows the pharmacy team to: All prescription items that have been referred back must be completed and returned to the NHSBSA no later than 18 months from the date they are first sent to the pharmacy. Pharmacy owners can also track the status of an unpaid item via the MYS portal: The MYS portal also allows users to generate reports to help pharmacy owners keep track of the status of any unpaid items. The reports can also be used as a learning tool to support staff training on the correct prescription endorsements to apply to avoid or minimise the risk of items being returned to the pharmacy for more information.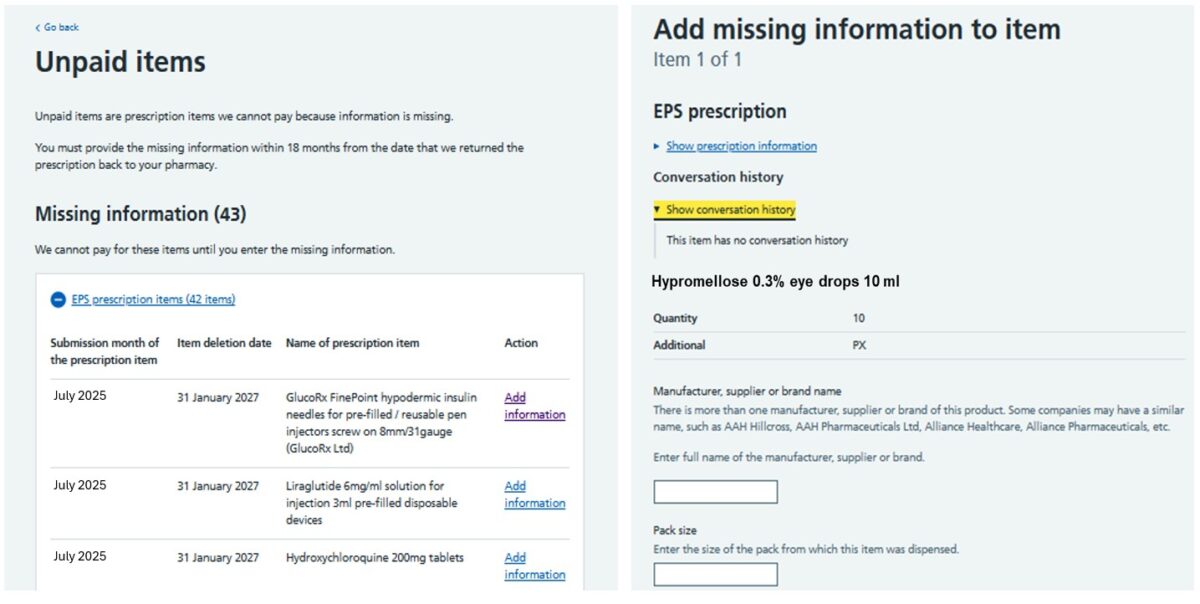
During the end of month submission process, the total number of completed referred back prescription forms and items being resubmitted should be declared in the relevant boxes in of the digital FP34C submission form. Please note only the number of items returned for clarification where payment has been delayed should be declared on the digital MYS submission form. This is because payment will already have been received for any other items on the returned prescription forms. On the digital MYS submission form:
Referred back items re-submitted for payment are priced using the Drug Tariff relevant to the dispensing month in which the NHSBSA confirm the item has been processed for payment. If the referred back item is not listed in the Drug Tariff, reimbursement will be based on the NHS dictionary of medicines and devices (dm+d) list price applicable for the month in which the unpaid item is received by the NHSBSA. If no dm+d list price is held by the NHSBSA, reimbursement will be based on pharmacy endorsed invoice price, if applicable. If the submitted information is still insufficient to allow NHSBSA to process the prescription for payment, the item will be re-sent to the pharmacy owner. Delays in the completion of missing information for referred back items can affect the timing of payments. This can create potential cash-flow risks for pharmacy owners if very expensive items are referred back, or a large number of items are returned to the pharmacy team for more information.
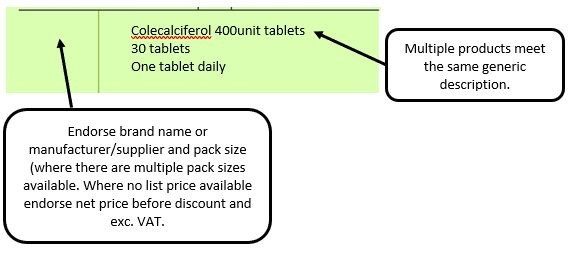
We have listed below examples of missing information and what is required to allow the item to be reimbursed. Example 1 – Supplier or manufacturer details missing – appliance Example 2 – Product name missing Example 3 – No pharmaceutical form listed on the prescription Action: If the pharmacy receive a prescription with the pharmaceutical form missing the prescription, If a prescription is presented with a missing presentation and the prescriber cannot be contacted, the presentation can be endorsed and dispensed if you have sufficient information to make a professional judgement. More information can be found here. If not, the prescription should be returned to the prescriber to be amended to include the pharmaceutical form. Example 4 – Total quantity not stated but clear from dosage instructions Example 5 – Endorsement of non-Part VIIIA generic products Example 6 – Missing prescriber code
The pharmacy team receive a generically written prescription for an appliance and there are multiple products listed in the appliances section of the Drug Tariff which meet that description.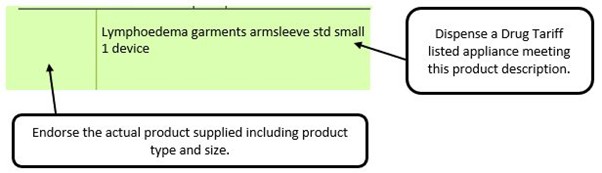
The pharmacy receive a generic prescription for an inhaler including a trademarked name (Accuhaler)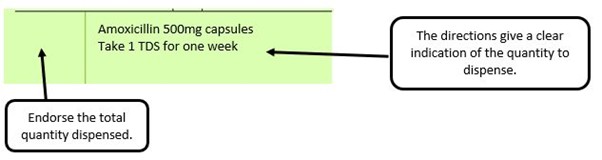
A disallowed item is one that has NOT been passed for payment by the NHSBSA. Disallowed items should not be confused with referred back items. Disallowed items are not passed for payment by the NHSBSA whereas referred back items are returned for further clarification to enable the NHSBSA to process the item for payment. Items issued on NHS prescription forms are disallowed by the NHSBSA for many reasons, some examples include where: The pricing systems flag such items to NHSBSA staff, who then re-check affected prescriptions and mark relevant items as disallowed, following Drug Tariff and prescription processing rules.
The NHSBSA apply a coding system to identify up to 19 possible reasons for disallowing items that are ordered on the NHS. Each prescription item disallowed by the NHSBSA is assigned a DA code indicate the reason for the disallowed item. The table below shows a list of Disallowed Codes which are applicable to community pharmacies.
Disallowed (DA) indicator
Disallowed reason
DA1
As certain drugs and other substances listed in the Drug Tariff Part XVIIIA cannot be allowed in these circumstances.
DA2
As it is not an appliance or chemical reagent listed in the Drug Tariff. See Drug Tariff Part I, Clause 2 and Clause 3.
DA3
As it is not a drug, appliance or chemical reagent listed in the Drug Tariff Part XVIIB(i) from which a Nurse Prescriber may order on prescription form FP10P.
DA4
As it is not a drug listed in the Drug Tariff Part XVIIA from which a Dental Practitioner may order on prescription.
DA6
As it is not a drug or preparation which may be prescribed on form FP10MDA in accordance with the NHS (General Medical Services Contracts) Regulations 2015 Paragraph 56 (10).
DA19
As this is not an authorised or valid form.
DA20
As it is a nominated EPS token submitted for processing and reimbursement.
Pharmacy owners will not receive payment for disallowed items, which can result in financial losses. For instance, dispensing a product that has recently been removed from the Appliances section (Part IX) of the Drug Tariff may lead to the pharmacy owner incurring losses. Implementing checks to ensure that only reimbursable prescriptions are dispensed can help prevent such losses.
The NHSBSA send a notification email to the pharmacy NHSmail account when a new disallowed item is generated. Pharmacy teams can access their disallowed items in the Manage your service (MYS) portal under the ‘Disallowed items’ tab. Disallowed items on the MYS portal are split into two groups: EPS and paper prescriptions. The MYS page will show details of the disallowed item including the month in which the item was originally submitted for payment, and the reason why the item was disallowed by the NHSBSA. The image below is an example of how a disallowed item appears in the MYS portal: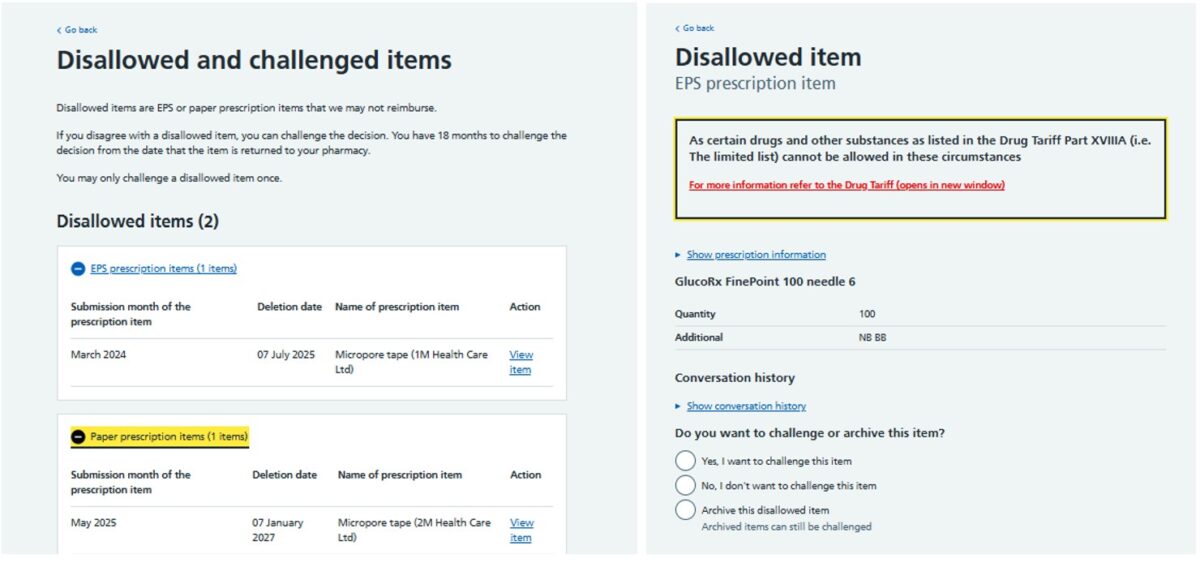
If it is believed that an item has been incorrectly disallowed by the NHSBSA, pharmacy owners may submit a challenge, via MYS, to the NHSBSA, who will investigate the issue and rectify any missing payments if a processing error is identified. To challenge disallowed items, pharmacy owners can follow the steps below: The NHSBSA will then investigate the disallowed item challenge and if a processing error is identified, the NHSBSA will adjust the payment accordingly. After challenging a disallowed item, the item will move to the challenged items section in the MYS portal and can either remain disallowed (if the NHSBSA has determined the item was correctly disallowed) or the NHSBSA may change the status of the item from disallowed to an ‘Unpaid item’ if further information is required to process payment. If enough information is present, the item will be processed for payment. It is important to note that disallowed items are only held on MYS for a period of 18 months from the date they are first sent to the pharmacy; if you have not reviewed and challenged any disallowed items before this deadline has passed, these items will be deleted from the system and the pharmacy will be unable to submit a challenge, if applicable. NHSBSA will notify pharmacy owners by email of the outcome of the challenge. If the challenge is unsuccessful, pharmacy owners may reply to the email to request further clarification or to request an appeal. If a pharmacy owner remains unsatisfied with the outcome of a disallowed item challenge, they can contact Community Pharmacy England’s Dispensing & Supply team on 0203 1220 810 or email their query to ds.team@cpe.org.uk for further advice on the matter.
Pharmacy owners can also check whether an item was referred back or disallowed by checking their monthly Prescription Item reports (PIRs). The product will be marked in column AP (column heading ‘RB’) if an item has been referred back. Column AQ (column heading ‘RB value’) displays the RB code for which the item was referred back. Similarly, the product will be marked in column W (column heading ‘DA’) if a product has been disallowed. Column X (column heading ‘DA value’) displays the reason code for the prescription being disallowed. To access item reports, pharmacy owners should register for the Information Services Portal on the NHSBSA’s website.
Pharmacy teams should also sign up to receive the quarterly NHSBSA Hints and Tips Bulletin to receive information, support and best practice guidance.
Q. NHSBSA has returned a photocopy of an FP10D dental prescription for ‘Ibuprofen 100mg/5ml oral suspension’ marked as disallowed. Why was this prescription not passed for payment? A. Only items listed in the Dental Practitioners’ Formulary (DPF) in Part XVIIA of the Drug Tariff can be prescribed on an FP10D prescription. There is a sugar-containing and sugar-free formulation of this product available. However, only the sugar-free formulation is listed in the DPF; therefore, only ‘Ibuprofen 100mg/5ml oral suspension sugar free’ can be prescribed on NHS dental prescriptions. If a pharmacy receives a FP10D for the standard ‘Ibuprofen 100mg/5ml oral suspension’, the prescription should be returned to the dentist for the words ‘sugar free’ to be added. Q. NHSBSA has returned a photocopy of a green FP10 prescription for Nytol 25mg tablets marked as disallowed. Why was this prescription not passed for payment? A. Nytol 25mg tablets is covered by the ’Nytol Tablets’ listing in Part XVIIIA of the Drug Tariff for ‘Drugs, Medicines and other Substances not to be ordered under a General Medical Services Contract’ and will therefore not be passed for payment by the NHSBSA if it issued on an NHS prescription form regardless of prescriber type. Q. I have a prescription for ‘Olive oil ear drops 10ml’, can I dispense Cerumol olive oil ear drops? A. No. Cerumol olive oil ear drops is classed as a medical device (as it has a CE mark) and would not be passed for payment because the item is not listed in the Appliances section in Part IXA of the Drug Tariff. Other brands or suppliers of olive oil ear drops are listed in Part IXA of the Drug Tariff which will be passed for payment, if endorsed accordingly. Q. How can I identify the prescribing rights of different types of nurse prescribers? A. To identify a nurses’ prescribing qualifications, pharmacy contractors are advised to check the register held by the Nursing & Midwifery Council. The qualifications can help determine what a nurse is able prescribe and if the prescription will be passed for payment. See our Who can prescribe what? page for information of the prescribing rights for different types of prescribers. Q. An item has been returned as disallowed because ‘it is not a drug, appliance or chemical reagent listed in the Drug Tariff Part XVIIB from which a Nurse Prescriber may order on a prescription form’. Can this disallowed item be challenged if the prescription was issued by a Nurse Independent Prescriber? A. Nurse Independent Prescribers are not restricted to prescribing items listed in the Nurse Prescribers’ Formulary (NPF) listed in Part XVIIB of the Drug Tariff. Nurse Independent Prescribers can prescribe any medicine (except for those listed in Part XVIIIA of the Drug Tariff) for any medical condition, within their own professional competence and expertise. This includes any Controlled Drugs listed in Schedules 2-5, except diamorphine, dipipanone, or cocaine for treating organic disease or injury but not for treating addiction. If an item prescribed by a Nurse Independent Prescriber has been incorrectly disallowed, pharmacies may submit a disallowed item challenge to the NHSBSA who will investigate the issue and rectify any missing payments if a processing error is identified. Q. I successfully challenged NHSBSA on an incorrectly disallowed prescription. Which months Drug Tariff will reimbursement be based on? A. If a successful challenge is made to a disallowed item, the product will be priced according to the month in which the item was re-processed by the NHSBSA for example, if a product was first submitted with a pharmacies January 2025 bundle and was successfully challenged in March 2025, the pricing of the prescription would be paid based on the March 2025 Drug Tariff. Q. Can I receive reports of items referred back to the pharmacy each month? A. Pharmacy owners can view reports on the MYS portal. The portal shows how many referred back items were received from the NHSBSA, how many have been re-submitted, how many are awaiting action by the NHSBSA and how many have been successfully processed. The reports help to keep track of RBs and can be used as a learning tool for pharmacy staff to help reduce the number of RBs by endorsing prescriptions correctly. Pharmacy owners can also check whether an item was referred back by checking their monthly Prescription Item reports (PIRs). The product will be marked in column AP (column heading ‘RB’) if an item has been RB. Column AQ (column heading ‘RB value’) displays the RB code for which the item was RB. To access item reports, contractors should register for the Information Services Portal on the NHSBSA’s website. Q. Do completed referred back items need to be declared on my digital FP34C MYS submission form? A. Yes, the total number of completed RB prescription forms and items being resubmitted should be declared in the relevant boxes on the digital MYS submission form. Please note only the number of items returned for clarification where payment has been delayed should be declared on the digital MYS submission form. This is because payment will already have been received for any other items on the returned forms. Q. If a prescription contains multiple items but only one item is referred back to the pharmacy, are all items on the prescription considered referred back awaiting payment? A. If only one item is referred back to the pharmacy for missing information, only that item is withheld from payment until the NHSBSA receive the required information.
See our Community Pharmacy England Briefing 020/22: Understanding prescription returns and disallowed items.
Related Resources
Sorting prescriptions for end of month submission
For more information on this topic please email comms.team@cpe.org.uk