Daily dispensing checks
Published on: 15th July 2013 | Updated on: 15th March 2022
Below is a checklist of actions recommended to take before submitting your bundle to the Pricing Authority at the end of the month, which could help to improve the accuracy of pricing.
Contractors should familiarise themselves with the prescription submission requirements and red separator inclusion criteria. For information on the changes to the end of month prescription sorting requirements and the red red separator inclusion criteria see the following pages:
Prescription submission requirements
Red Separators – end of month submission
DO |

*For instance, Drug Tariff listed lines only require endorsement if they are a Category C item that comes in more than one pack size. Please see our endorsing guidance for more information. |
DO NOT |
 |
FAQs
Q. How do I put EPS Release 2 Prescriptions into the red separator? What do I do with red separator items on EPS Release 2?
A. There is no virtual red separator for EPS Release 2 prescriptions. The items on paper prescriptions that are eligible to go in the red separator should be priced accordingly on electronic prescriptions. However, Community Pharmacy England does advise that as part of the pharmacy’s reconciliation process, contractors should keep a log or generate report(s) from their PMR system for the following types of items:
- Expensive items (items with a net ingredient cost of £100 or over);
- Specials and unlicensed products; and
- Items with broken bulk or out of pocket expense claims.
This report or log should then be used to reconcile items/values on the pharmacy’s FP34 Schedule of Payment which is sent by the Pricing Authority after the bundle has been priced. If you are unable or unsure as to how to generate a report which incorporates the above, contact your PMR system supplier for further information.
Pharmacy teams are also reminded that EPS tokens should not be placed in the red separator in the end of month prescription bundle. This is because these tokens are not used for payment of electronic prescriptions (payment is solely based on the information contained in the electronic claim message).[/showhide]
Q. Where I have sorted broken bulk items, items with a NIC of £100 or more and ‘Specials’ into the red separators for submission to the Pricing Authority, do I need to sort these in prescriber order?
A. No, there is no need to sort the broken bulk, items with a NIC of £100 or more and ‘Specials’ prescriptions in prescriber order.
However, broken bulk, items with a NIC of £100 or more and ‘Specials’ prescriptions must be sorted into the correct ‘charge paid’ and ‘exempt’ red separator and included on the top of the ‘charge paid’ or ‘exempt bundles’, as appropriate. There is no need to further separate out the broken bulk prescriptions from the ‘Specials’ prescriptions and ‘Items with a NIC of £100’.
Q. When preparing my prescriptions for submission to the Pricing Authority, do I have to sort my forms in to prescriber order?
A. No. Following representations made by Community Pharmacy England, the Department of Health and Social Care (DHSC) have agreed to relax the end of month prescription sorting requirements by removing the need for contractors to sort FP10 forms by prescriber surname/type or by form type (FP10SS PN/SP/HP and FP10D).
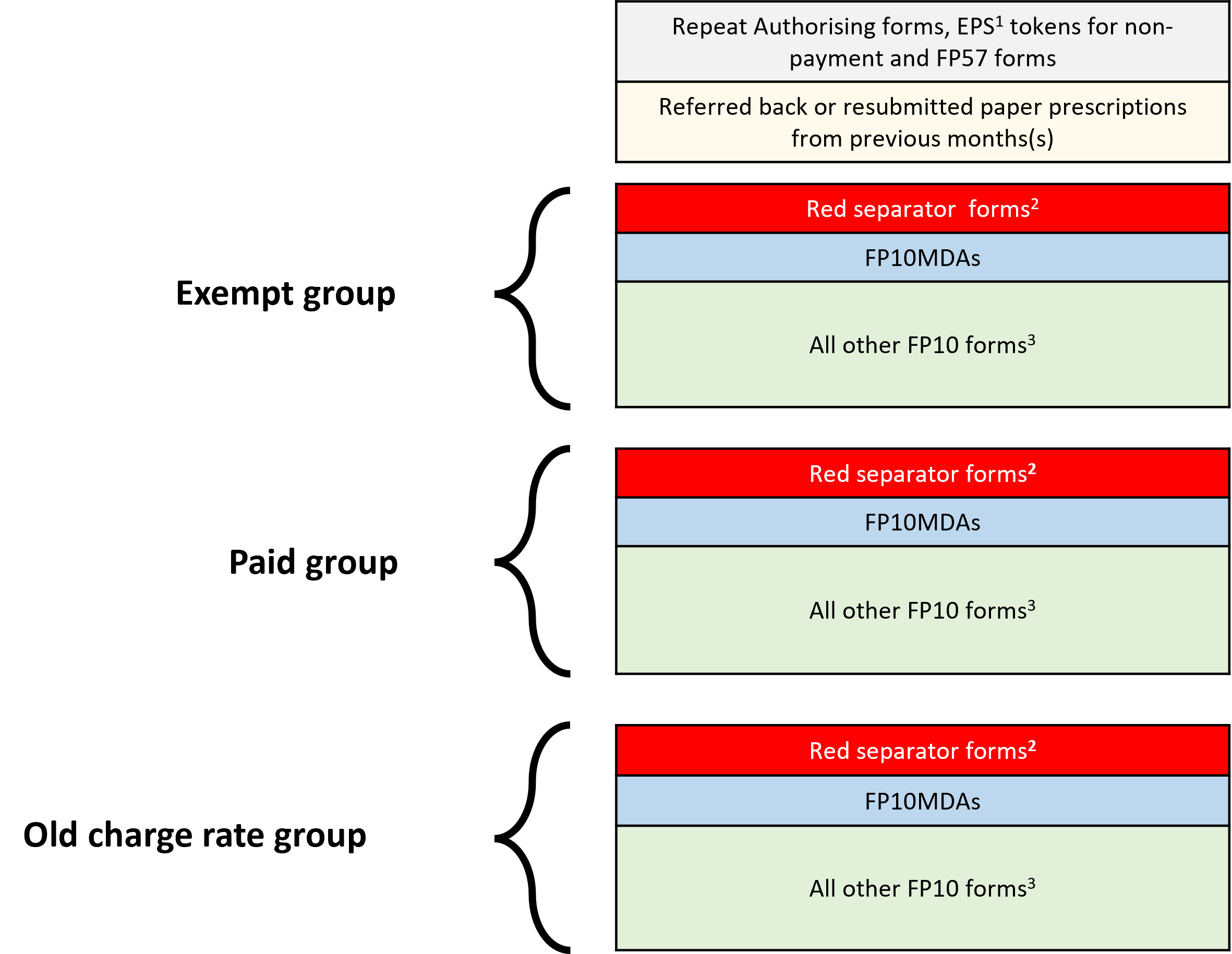
Pharmacy staff involved in end of month submission process will continue to be required to sort prescription forms into relevant exempt or paid groups (current rate and old charge rates) and use red separators for forms which include specified items before the prescription bundle is submitted for payment to the NHSBSA.
The recommended order for sorting of prescriptions is outlined below.
- EPS tokens for non-payment, FP57 forms and paper Repeat Authorising (RA) forms
- Resubmitted forms from previous month(s)
- Red separator forms (includes the newly added Bulk and HMP Service-issued prescriptions)
- FP10MDA instalment dispensing forms (where possible sorted open and flat)
- All other FP10 forms (sorting no longer required by form type (FP10SS PN/SP/HP and FP10D) or by prescriber surname)
The image below shows the correct prescription bundle sorting requirements:
1. Unless an automatic exemption applies, it is a condition of entitlement to exemption from prescription charges that the patient or their representative claiming exemption must make and duly complete a declaration of entitlement on the EPS token. Each month, contractors must submit tokens for electronic prescriptions (except those for age exempt patients, prescriptions where only free-of-charge (FOC) items are prescribed and where Real Time Exemption Checking (RTEC) confirms an exemption). Tokens must be sorted separately from FP10 paper prescriptions and dispatched with the prescription bundle no later than the 5th of the month following that in which supply was made. Where submitted, the EPS tokens must relate to the same the dispensing month in which the associated electronic prescriptions are submitted for payment to the NHSBSA
2. See Community Pharmacy England’s Red Separator factsheet for guidance on which forms are required to be placed in the red separators
3. Contractors are no longer required to sort FP10 prescriptions by form type (FP10SS PN/SP/HP and FP10D) or by prescriber name/type
Note: There are different submission arrangements for private Controlled Drug prescription forms (FP34PCD) and Controlled Drug requisition forms (FP10CDF) (see our Controlled Drugs section for more information).
Q.I have received a prescription for a product but the patient has requested less than the prescribed quantity. Do I need to sort this prescription differently for submission to the Pricing Authority?
A. No. The only prescriptions that have to be sorted differently are prescriptions containing broken bulk claims, items with a NIC of £100 or more and ‘Specials’.
In this scenario, to guard against any suggestion of fraud, the prescription should be clearly endorsed in the endorsement column to indicate the dispensed quantity. Although information in the endorsement column may not be picked up in the pricing process, the Pricing Authority has not asked contractors to separate out prescriptions where the dispensed quantity is less than the prescribed quantity.
Related resources
Claiming for EPS prescriptions on time factsheet
Prescription submission requirements
Red Separators – end of month submission
Dispensing Factsheet: Expensive items
For more information on this topic please email comms.team@cpe.org.uk